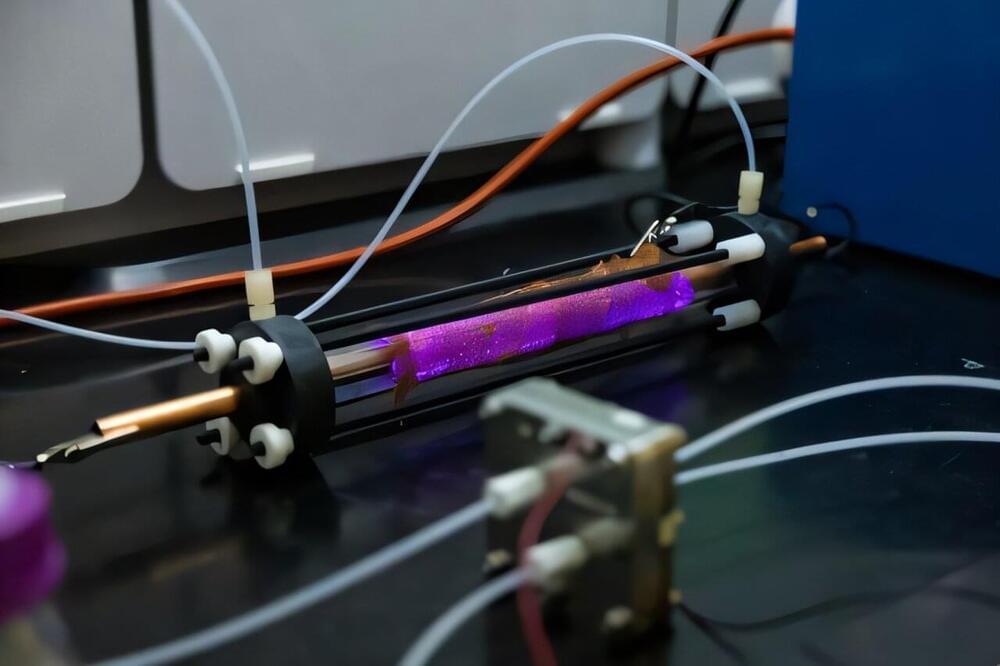
There’s a good chance you owe your existence to the Haber-Bosch process.
This industrial chemical reaction between hydrogen and nitrogen produces ammonia, the key ingredient in synthetic fertilizers that supply much of the world’s food supply and enabled the population explosion of the last century.
It may also threaten the existence of future generations. The process consumes about 2% of the world’s total energy supply, and the hydrogen required for the reaction mostly comes from fossil fuels.









Leave a reply