Researchers in Spain have devised a hybrid device that can generate energy using sunlight as well as raindrops.



Researchers have developed a solar cell system that uses mirrors to concentrate solar energy. In addition to electricity, it produces heat for a plant that will capture carbon from industrial emissions. The solar cells in the large pilot plant are a full 5 meters high and consist of many mirrors that are angled toward the solar cells to concentrate sunlight. They make it possible to collect the sun’s rays into concentrated solar energy, as well as heat that supports a plant designed to capture CO2.
“The system has been tested and validated. It is quite innovative and unique and stands out by storing heat in addition to the electrical current,” says SINTEF research scientist Alfredo Sanchez Garcia.
The energy from the plant will be used to capture carbon from industrial emissions.
A study conducted by Penn State University researchers has revealed that organic solar cells could be strengthened by adding a chemical additive, making them suitable for large-scale deployment and manufacturing. The study was reported on the official university website on February 16.
Assistant Professor Nutifafa Doumon and doctoral candidate Souk Yoon “John” Kim, both from the Department of Materials Science and Engineering, led this experiment.


Despite being riddled with impurities and defects, solution-processed lead-halide perovskites are surprisingly efficient at converting solar energy into electricity. Their efficiency is approaching that of silicon-based solar cells, the industry standard. In a new study published in Nature Communications, physicists at the Institute of Science and Technology Austria (ISTA) present a comprehensive explanation of the mechanism behind perovskite efficiency that has long perplexed researchers.
How can a device assembled with minimal sophistication rival state-of-the-art technology perfected over decades? Over the past 15 years, materials research has witnessed the rise of lead-halide-based perovskites as prospective next-generation solar-cell materials. The puzzle is that despite similar performance, perovskite solar cells are fabricated using inexpensive solution-based techniques, while the industry-standard silicon cells require ultra-pure single-crystal wafers.
Now, postdoc Dmytro Rak and assistant professor Zhanybek Alpichshev at the Institute of Science and Technology Austria (ISTA) have uncovered the mechanism behind the unique photovoltaic properties of perovskites. Their key finding is that while silicon-based technology relies on the absence of impurities, the opposite is true in perovskites: It is the natural network of structural defects in these materials that enables the long-range charge transport necessary for efficient photovoltaic energy harvesting.
Extremely cool paper describing optically programmable ~0.3 mm robots with onboard computation and autonomous locomotion! These tiny rectangular machines carry solar cells, optical receivers, electrokinetic actuators, and more. As demonstrations, the authors programmed them (i) to report local temperature by doing a coded dance and (ii) swim towards warmth before stopping and rotating upon reaching a location with a certain level of heat. This is amazing and I hope such devices are further improved so they can be used in biological applications! Love it!
(https://www.science.org/doi/10.1126/scirobotics.adu8009)
Autonomous submillimeter robots are built with onboard sensing, computation, memory, communication, and locomotion.

When the sun goes down, solar panels stop working. This is the fundamental hurdle of renewable energy: how to save the sun’s power for a rainy day—or a cold night. Chemists at UC Santa Barbara have developed a solution that doesn’t require bulky batteries or electrical grids. In a paper published in the journal Science, Associate Professor Grace Han and her team detail a new material that captures sunlight, stores it within chemical bonds and releases it as heat on demand.
The material, a modified organic molecule called pyrimidone, is the latest advancement in molecular solar thermal (MOST) energy storage.
“The concept is reusable and recyclable,” said Han Nguyen, a doctoral student in the Han Group and the paper’s lead author.

In a bold fusion of SpaceX’s satellite expertise and Tesla’s AI prowess, the Starthink Synthetic Brain emerges as a revolutionary orbital data center.
Proposed in Digital Habitats February 2026 document, this next-gen satellite leverages the Starlink V3 platform to create a distributed synthetic intelligence wrapping the planet.
Following SpaceX’s FCC filing for up to one million orbital data centers and its acquisition of xAI, Starthink signals humanity’s leap toward a Kardashev II civilization.
As Elon Musk noted in February 2026, ]
“In 36 months, but probably closer to 30, the most economically compelling place to put AI will be space.”
## The Biological Analogy.
Starthink draws from neuroscience: * Neural Cluster: A single Tesla AI5 chip, processing AI inference at ~250W, like a neuron group. * Synthetic Brain: One Starthink satellite, a 2.5-tonne self-contained node with 500 neural clusters, solar power, storage, and comms. * Planetary Neocortex: One million interconnected Brains forming a global mesh intelligence, linked by laser and microwave “synapses.”
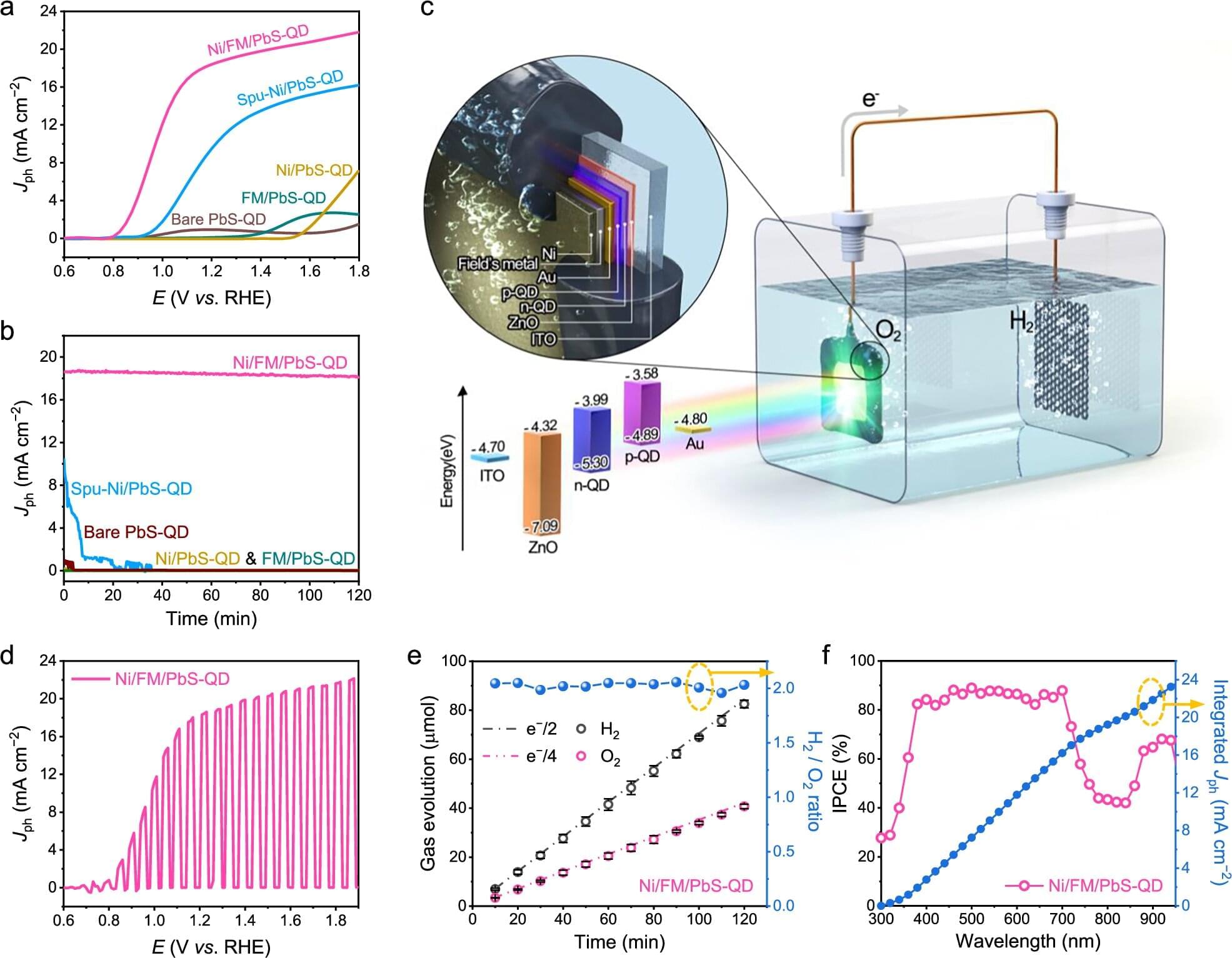
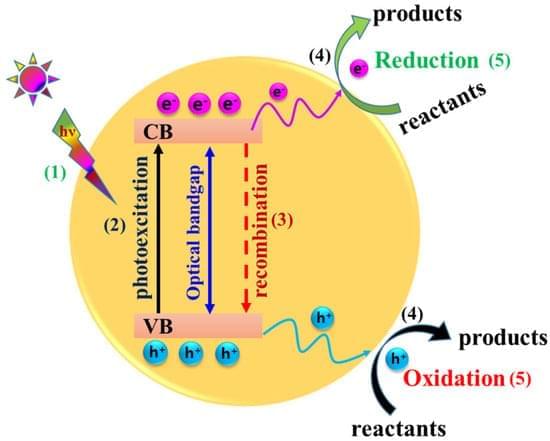
A research team affiliated with UNIST has developed stable and efficient chalcogenide-based photoelectrodes, addressing a longstanding challenge of corrosion. This advancement paves the way for the commercial viability of solar-driven water splitting technology—producing hydrogen directly from sunlight without electrical input.
Jointly led by Professors Ji-Wook Jang and Sung-Yeon Jang from the School of Energy and Chemical Engineering, the team reported a highly durable, corrosion-resistant metal-encapsulated PbS quantum dot (PbS-QD) solar cell-based photoelectrode that delivers both high photocurrent and long-term operational stability for photoelectrochemical (PEC) water splitting without the need for sacrificial agents. The research is published in the journal Nature Communications.
PEC water splitting is a promising route for sustainable hydrogen production, where sunlight is used to drive the decomposition of water into hydrogen and oxygen within an electrolyte solution. The efficiency of this process depends heavily on the stability of the semiconductor material in the photoelectrode, which absorbs sunlight and facilitates the electrochemical reactions. Although chalcogenide-based sulfides, like PbS are highly valued for their excellent light absorption and charge transport properties, they are prone to oxidation and degradation when submerged in water, limiting their operational stability.