“CO2 capture with post-modified nitrile-and styrene-butadiene-styrene rubbers”
Chem.
This website uses a security service to protect against malicious bots. This page is displayed while the website verifies you are not a bot.



“If you don’t know exactly how much emissions you have, then it’s really difficult to make effective policies or technologies or methods to reduce the emissions,” said Dr. Z. Jason Ren. [ https://www.labroots.com/trending/earth-and-the-environment/…-reports-2](https://www.labroots.com/trending/earth-and-the-environment/…-reports-2)
Are national climate reports missing crucial data points regarding wastewater greenhouse gas (GHG) emissions? This is what a recent study published in Nature Climate Change hopes to address as a team of researchers investigated the accuracy of national inventory reports (NIRs) for wastewater GHG. This study has the potential to help researchers, climate scientists, legislators, and the public better understand the methods for tracking climate change and steps that can be taken to fill the gaps in report lapses.
For the study, the researchers obtained data from 38 countries regarding wastewater GHG emissions with the goal of ascertaining existing data gaps in NIRs. The motivation of this study comes from the lack of consistent data methods and large changes that occur over many years and in global regions. The overarching goal of the study was to ascertain where the data gaps exist and how to fill them.
In the end, the researchers discovered massive data gaps in wastewater GHG emissions, including an unreported gap of 52.0–73.2 million metric tons (MMT) of CO2-equivalent (CO2e) annually across the 38 countries. Additionally, they found that global gap of 94–150 MMT CO2e annually.

Fifteen years ago, I wrote something that annoyed many techno-optimists.
Ten years ago, I filmed it as a podcast.
Today it feels less controversial — and more urgent.
Technology is NOT Enough.
We have the science to feed everyone. We have the tech to provide clean water. We understand climate change. We know how to reduce suffering.
And yet we don’t act.

A new material can store energy from sunlight and convert it into hydrogen days later. The material, jointly developed by researchers from Ulm and Jena, can do this even in the dark. The process is reversible and can be reactivated several times using a pH switch. The results are published in the journal Nature Communications.
Green hydrogen is one of the most important pillars of the energy transition. It is produced from sunlight using photocatalytic processes. There are now a variety of technologies for converting and storing solar energy into chemical energy. But now, for the first time, a material that can store the energy from sunlight for several days and then release it in the form of hydrogen “at the push of a button” has been successfully developed.
“You can think of it as a combination of a solar cell and a battery at the molecular level,” explains Professor Sven Rau, who heads the Institute of Inorganic Chemistry I at Ulm University.
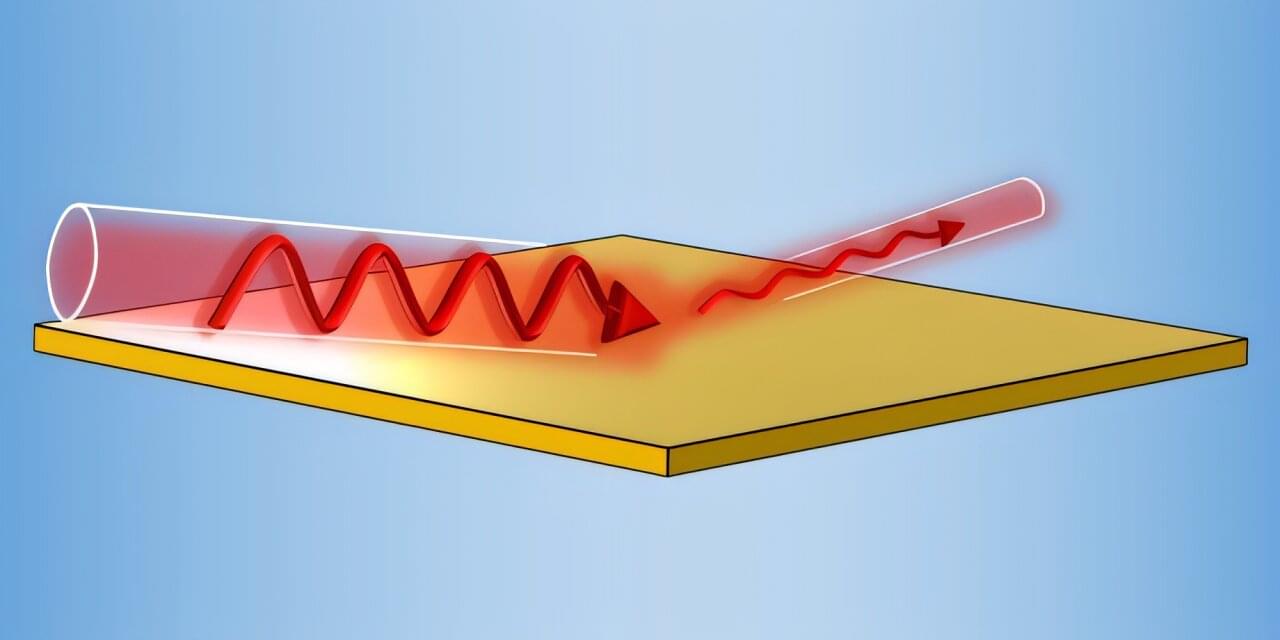
The applications of ultrathin, conductive films such as those made of graphene have many applications, but it’s been thought their efficacy is limited to absorbing only half of the incidental light at best. A research group in China has now shown that absorption can be as high as 82.8% at light grazing angles nearly parallel to the film. This could not only significantly improve design efficiencies but sheds light on light-matter interactions at sizes much lower than the light’s wavelength. Their work has been published in Physical Review Letters.
Graphene ultrathin films, as thin as one carbon atom (about 0.34 nanometers, 300,000 times thinner than a sheet of paper) have many applications: flexible and transparent electronics, energy storage and batteries, solar cells and photovoltaics, sensors and high-speed electronics and more, where they absorb light.
While such films allow for miniaturizing devices and reducing their weight, their extreme thinness has led to the characterization that they are limited to absorbing only half of the incoming light.


Chinese scientists have developed a lithium metal battery that boasts an energy density of more than 700 watt-hours per kilogram and stable performance at extremely low temperatures, marking a significant advancement in the production of high-energy batteries for electric vehicles. The research paper was published on Thursday in the science journal Nature.
Chen Jun, an academician of the Chinese Academy of Sciences and vice-president of Nankai University in Tianjin, was among the researchers who led the breakthrough. Chen said the team has replaced oxygen atoms with fluorine ones. It designed and synthesized novel fluorinated hydrocarbon solvent molecules, creating a new electrolyte system based on lithium-fluorine coordination.

Contamination of ground, surface and drinking water by perfluoroalkyl and polyfluoroalkyl substances (PFAS) affects millions of people worldwide. A promising new method developed by Flinders University scientists paves the way to help remove the most difficult-to-capture variants of these persistent pollutants from water.
The research team, led by Flinders ARC Research Fellow Dr. Witold Bloch, has discovered adsorbents that effectively capture PFAS, including short-chain forms that are especially difficult to remove using existing technologies.
The study, published in the Angewandte Chemie International Edition, showcases the use of a nano-sized molecular cage that acts as a highly selective “PFAS trap.”
