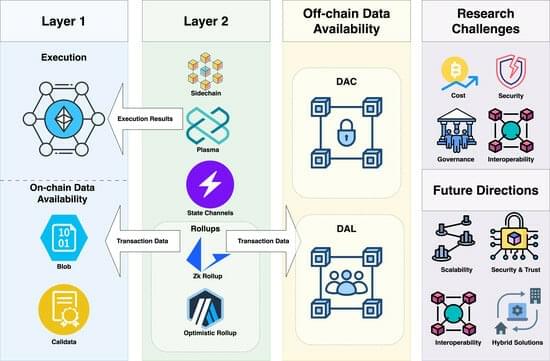
The rapid advancement of technologies, particularly AI, is driving the world towards an economic singularity where the marginal cost of essentials approaches zero, leading to a deflationary future and a potential transformation of traditional systems and societies ##
## Questions to inspire discussion.
Education Transformation.
🎓 Q: How will AI reduce education time while improving effectiveness?
A: AI will customize education to each child’s learning style, reducing daily learning time to 1 hour per day while delivering 5 times more effective learning compared to traditional methods, with costs falling to zero within 3–5 years and breaking the university industry that currently creates massive student debt.
Healthcare Revolution.