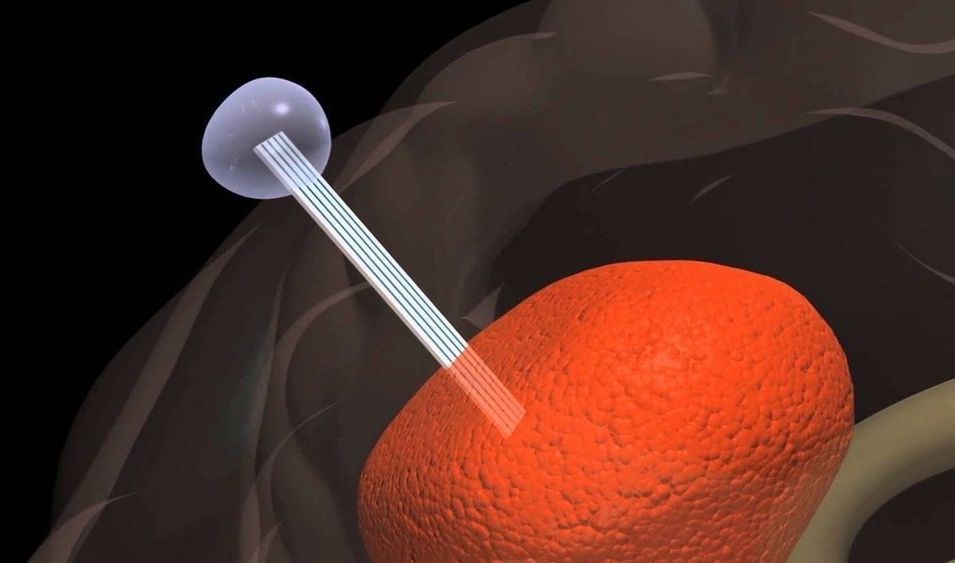
A biomedical tool that tricks aggressive brain tumors such as glioblastoma into migrating into an external container rather than throughout the brain has been designated a “Breakthrough Device” by the U.S. Food and Drug Administration (FDA).
Dubbed the Tumor Monorail, the device mimics the physical properties of the brain’s white matter to entice aggressive tumors to migrate toward the exterior of the brain, where the migrating cells can be collected and removed. The purpose of the device is not to destroy the tumor, but to halt its lethal spread, making the disease more of a condition to manage than a death sentence.
Breakthrough designations from the FDA aim to expedite the development and review of drugs, diagnostics and devices aimed at life-threatening or irreversibly debilitating conditions. While the designation does not mean that the device has been approved for clinical use, it does provide a partnership with the FDA that can speed development, assessment and review.









Comments are closed.