Amazon is selling a slew of tiny homes on its Website for different price points including ones that are under $20,000.




BEIJING — China has turned its western region of Xinjiang into a police state with few modern parallels, employing a combination of high-tech surveillance and enormous manpower to monitor and subdue the area’s predominantly Muslim ethnic minorities.
Now, the digital dragnet is expanding beyond Xinjiang’s residents, ensnaring tourists, traders and other visitors — and digging deep into their smartphones.
A team of journalists from The New York Times and other publications examined a policing app used in the region, getting a rare look inside the intrusive technologies that China is deploying in the name of quelling Islamic radicalism and strengthening Communist Party rule in its Far West. The use of the app has not been previously reported.
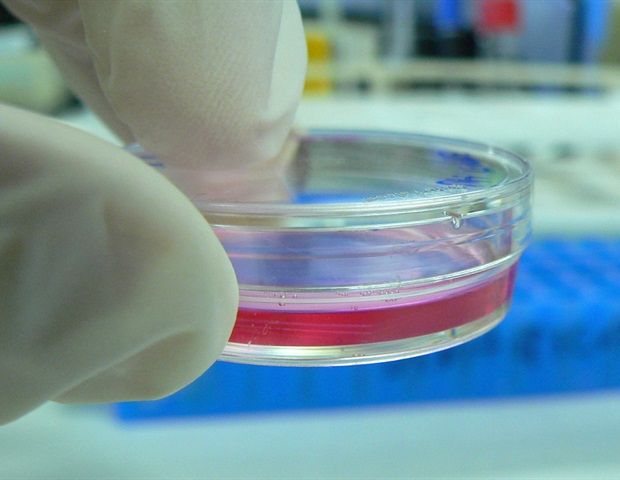
The loss of complete segments of the esophagus often results from treatments for esophageal cancer or congenital abnormalities, and current methods to re-establish continuity are inadequate. Now, working with a rat model, researchers have developed a promising reconstruction method based on the use of 3D-printed esophageal grafts. Their work is published in Tissue Engineering, a peer-reviewed journal from Mary Ann Liebert, Inc., publishers.
Eun-Jae Chung, MD, PhD, Seoul National University Hospital, Korea, Jung-Woog Shin, PhD, Inje University, Korea, and colleagues present their research in an article titled “Tissue-Engineered Esophagus via Bioreactor Cultivation for Circumferential Esophageal Reconstruction”. The authors created a two-layered tubular scaffold with an electrospun nanofiber inner layer and 3D-printed strands in the outer layer. After seeding human mesenchymal stem cells on the inner layer, constructs were cultured in a bioreactor, and a new surgical technique was used for implantation, including the placement of a thyroid gland flap over the scaffold. Efficacy was compared with omentum-cultured scaffolding technology, and successful implantation and esophageal reconstruction were achieved based on several metrics.
Dr. Chung and colleagues from Korea present an exciting approach for esophageal repair using a combined 3D printing and bioreactor cultivation strategy. Critically, their work shows integration of the engineered esophageal tissue with host tissue, indicating a clinically viable strategy for circumferential esophageal reconstruction.”

When they aren’t investing in space shuttles and sprawling tech campuses, the super-rich are looking at mind-blowing methods to increase their lifespan.
Analysis by commercial finance experts ABC Finance has revealed some of the strangest and most extravagant approaches billionaires have turned to in their quest for immortality.
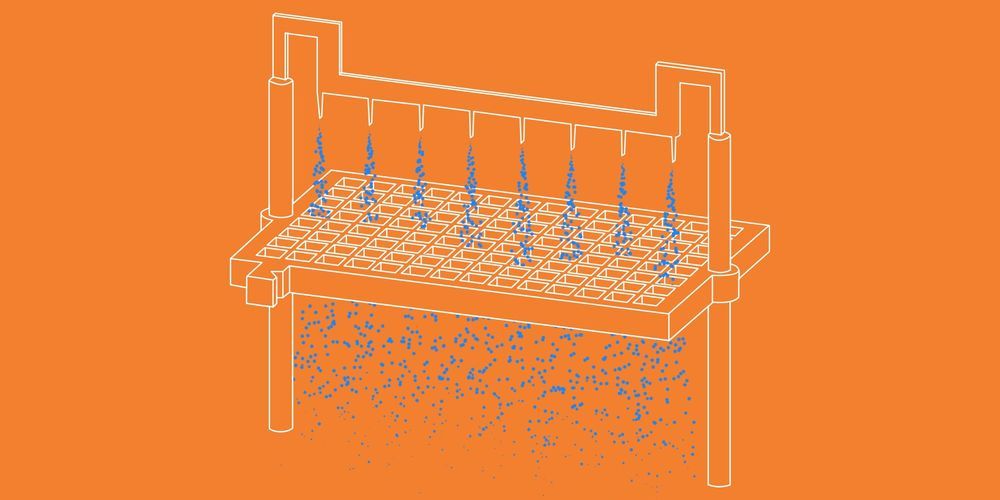
Credit where credit is due: Evolution has invented a galaxy of clever adaptations, from fish that swim up sea cucumber butts and eat their gonads, to parasites that mind-control their hosts in wildly complex ways. But it’s never dreamed up ion propulsion, a fantastical new way to power robots by accelerating ions instead of burning fuel or spinning rotors. The technology is in very early development, but it could lead to machines that fly like nothing that’s come before them.
You may have heard of ion propulsion in the context of spacecraft, but this application is a bit different. Most solar-powered ion spacecraft bombard xenon atoms with electrons, producing positively charged xenon ions that then rush toward a negatively charged grid, which accelerates the ions into space. The resulting thrust is piddling compared to traditional engines, and that’s OK—the spacecraft is floating through the vacuum of space, so the shower of ions accelerate the aircraft bit by bit.
You’ve read your last complimentary article this month. To read the full article, SUBSCRIBE NOW. If you’re already a subscriber, please sign in and and verify your subscription.
Coming into effect as early as 2021.
