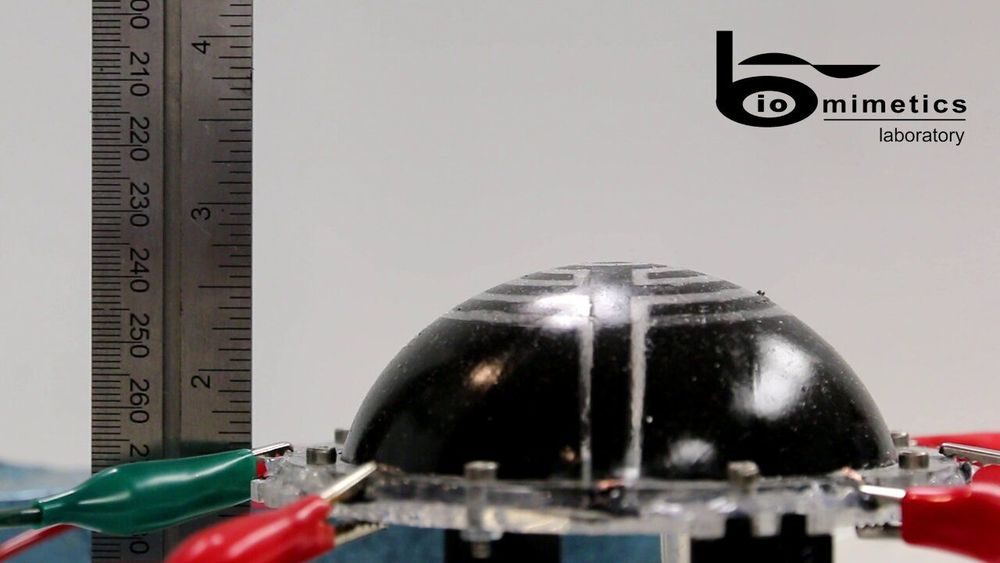
Researchers at the Auckland Bioengineering Institute and Technische Universit\xE4t Dresden have recently designed a new type of inflatable robot for space navigation. These robots, presented in a paper published in SPIE Digital Library, were created using dielectric elastomer transducers (DETs), which are essentially electrical capacitors made from soft rubbery materials.
“Current space technology is limited by its mass and volume. It takes thousands of dollars to launch even a single kilogram into orbit,” Joseph Ashby, one of the researchers who carried out the study, told TechXplore. “Our research aims to replace or augment current technology with lighter smart-material replacements combined with inflatable structures.”
If they are integrated with inflatable structures, DETs could aid the development of soft and low-mass robots, which have high packaging efficiency and are easy to deploy. In fact, DETs deform when a voltage is applied to them, due to the Maxwell stress generated by the electric field.