Astronomers have recently discovered the most massive neutron star to date, nearly at the theoretical limit for such stars. But it’s only about the size of a small city.

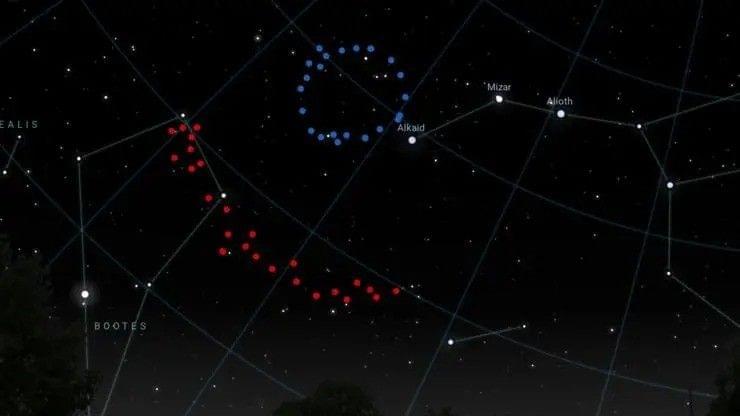
But by analyzing data taken from the Sloan Digital Sky Survey, which studies galaxies illuminated by powerful quasars bursts, the researchers teased apart the evidence for a ring far bigger than the theoretical upper size limit — a stunning coil-like structure aligned face-on with Earth.
“The Big Ring and Giant Arc are the same distance from us, near the constellation of Boötes the Herdsman, meaning they existed at the same cosmic time when the universe was only half of its present age,” Lopez said. “They are also in the same region of sky, at only 12 degrees apart when observing the night sky … [This] raises the possibility that together they form an even more extraordinary cosmological system.”
Although the cause of the gigantic structure is unclear, the researchers first speculated that it could be a remnant of a baryon acoustic oscillation (BAO), a type of sound wave that rippled through the hot plasma of the early universe. Yet further analysis found that the Big Ring was too large and, due to its corkscrew shape, not spherical like BAOs. Alternative explanations suggest that it could possibly be a cosmic string, a hypothetical clumping of matter created in the early universe, or a remnant of something else that could demand an entirely new model to explain it.
Now, astronomers led by Northwestern University have pinpointed the extraordinary object’s birthplace — and it’s rather curious, indeed.
Using images from NASA ’s Hubble Space Telescope, the researchers traced the FRB back to not one galaxy but a group of at least seven galaxies. The galaxies in the collection appear to be interacting with one another — perhaps even on the path to a potential merger. Such groups of galaxies are rare and possibly led to conditions that triggered the FRB.
The unexpected finding might challenge scientific models of how FRBs are produced and what produces them.
For every proton, there were over a billion others that annihilated away with an antimatter counterpart. So where did all that energy go?
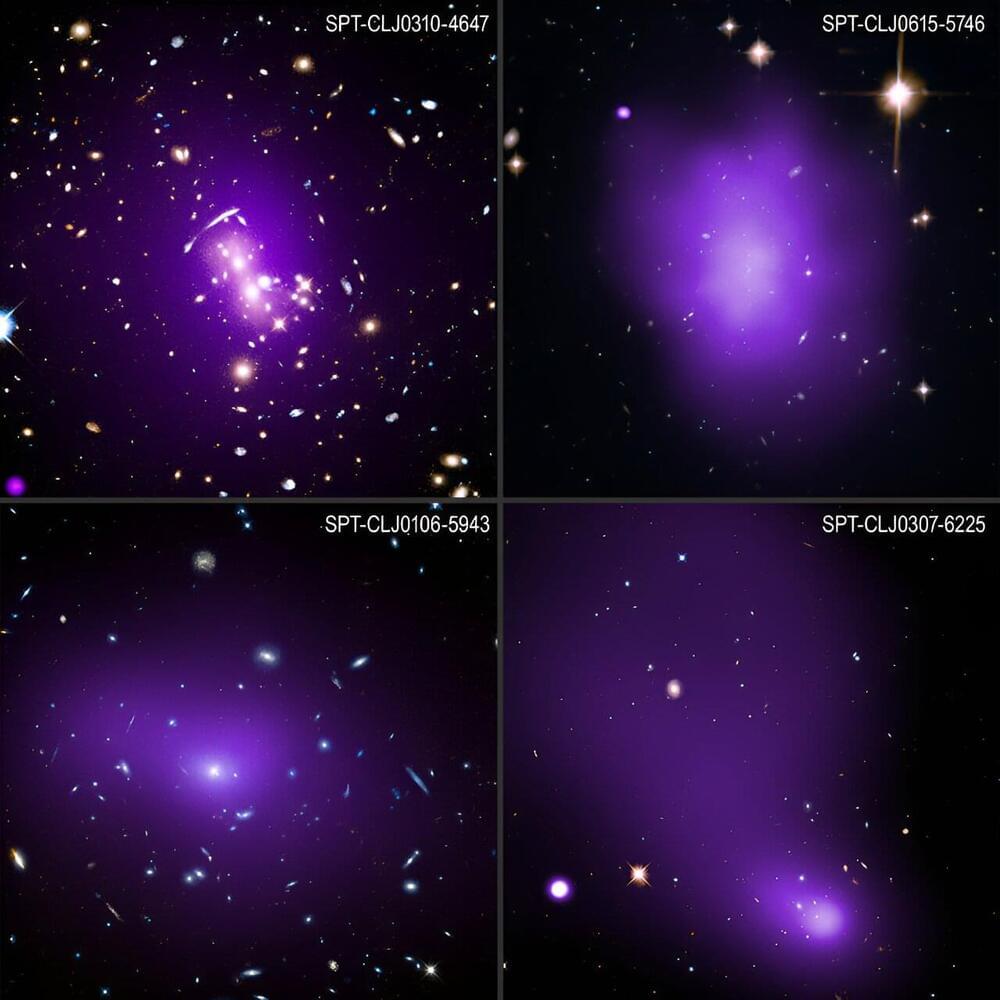
Astronomers have completed the largest and most detailed study of what triggers stars to form in the universe’s biggest galaxies, using NASA’s Chandra X-ray Observatory and other telescopes. They were surprised to find that the conditions for stellar conception in these exceptionally massive galaxies have not changed over the last ten billion years.
“What’s surprising here is that there are lots of things that could have affected star formation over the last ten billion years,” said Michael Calzadilla of the Massachusetts Institute of Technology (MIT) who led the study. “In the end, however, the main driver of star formation in these huge galaxies really comes down to one thing—whether or not the hot gas surrounding them can cool off quickly enough.”
Clusters of galaxies are the largest objects in the universe held together by gravity and contain huge amounts of hot gas seen in X-rays. The mass of this hot gas is several times the total mass of all the stars in all the hundreds of galaxies typically found in galaxy clusters.

While NASA has long been known for its space-related initiatives, the space agency also has a rich history of aeronautics research and development with high-speed aircraft. This makes today’s unveiling of the experimental X-59 quiet supersonic aircraft, a joint venture between NASA and defense contractor, Lockheed Martin, much more exciting. With the X-59, NASA hopes to collect data with the goal of revolutionizing commercial air travel, as the most well-known supersonic passenger aircraft was the Concorde, which retired in 2003.
“This is a major accomplishment made possible only through the hard work and ingenuity from NASA and the entire X-59 team,” said NASA Deputy Administrator Pam Melroy. “In just a few short years we’ve gone from an ambitious concept to reality. NASA’s X-59 will help change the way we travel, bringing us closer together in much less time.”
The X-59 aircraft is the centerpiece of NASA’s Quesst mission and stands for “Quiet Supersonic Technology”. The goal of Quesst is to develop supersonic aircraft that don’t produce the familiar sonic booms that supersonic aircraft are known to make when they break the sound barrier, which has been known to result in broken windows and significant noise pollution for civilian populations.

Astronomers discovered a mysterious gamma-ray feature outside our galaxy by analyzing 13 years of data from NASA’s Fermi Gamma-ray Space Telescope.
“It is a completely serendipitous discovery,” said Alexander Kashlinsky, a cosmologist at the University of Maryland and NASA’s Goddard Space Flight Center in Greenbelt, who presented the research at the 243rd meeting of the American Astronomical Society in New Orleans. “We found a much stronger signal in a different part of the sky than the one we were looking for.”
The gamma-ray signal is unexpected and intriguingly similar to another unexplained feature produced by some of the most energetic cosmic particles ever detected. A paper describing the findings is published in The Astrophysical Journal Letters.

WASHINGTON — SpaceWERX, the technology arm of the U.S. Space Force, is looking to award a new round of Small Business Innovation Research contracts worth up to $1.9 million each for IT infrastructure upgrades at the Eastern and Western launch ranges.
The project known as Digital Spaceport of the Future was announced earlier this month. SpaceWERX officials on Jan. 10 said launch ranges are in dire need of IT upgrades and are seeking proposals from the private sector by February 7.
Maj. Jareth Lamb, deputy director of SpaceWERX, said during a briefing that the contracts will be “direct to Phase 2” SBIR/STTR agreements. These are Small Business Innovation Research/Small Business Technology Transfer deals that require collaboration between small businesses and non-profit research institutions.
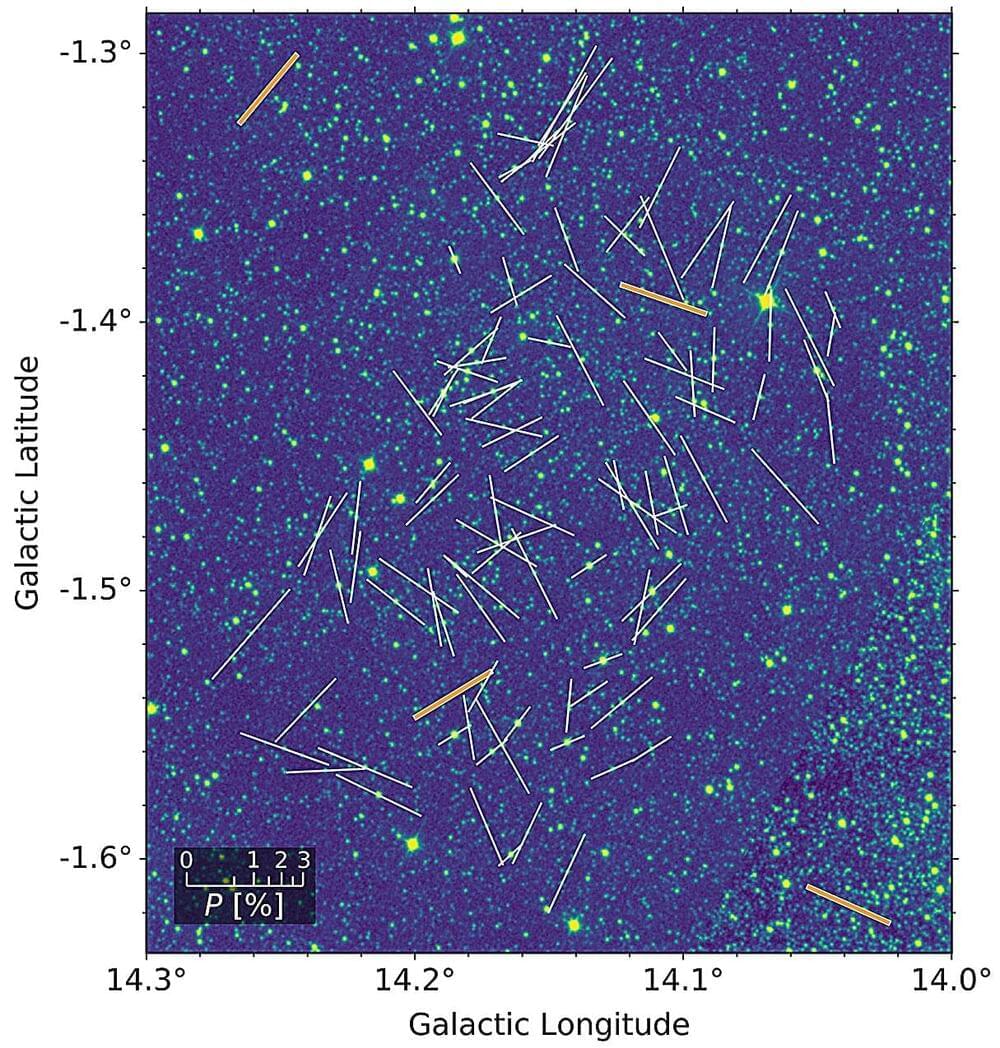
A team of astronomers including those from the University of Tokyo have created the first-ever map of magnetic field structures within a spiral arm of our Milky Way galaxy. Previous studies on galactic magnetic fields only gave a very general picture, but the new study reveals that magnetic fields in the spiral arms of our galaxy break away from this general picture significantly and are tilted away from the galactic average by a high degree.
The findings, appearing in The Astrophysical Journal, suggest magnetic fields strongly impact star-forming regions which means they played a part in the creation of our own solar system.
It might come as a surprise to some that magnetic fields can exist on scales larger than a planet. Most of our daily experience with magnetic fields involves either sticking things to our refrigerator, or perhaps using a compass to point north. The latter shows the existence of magnetic fields generated by our planet.
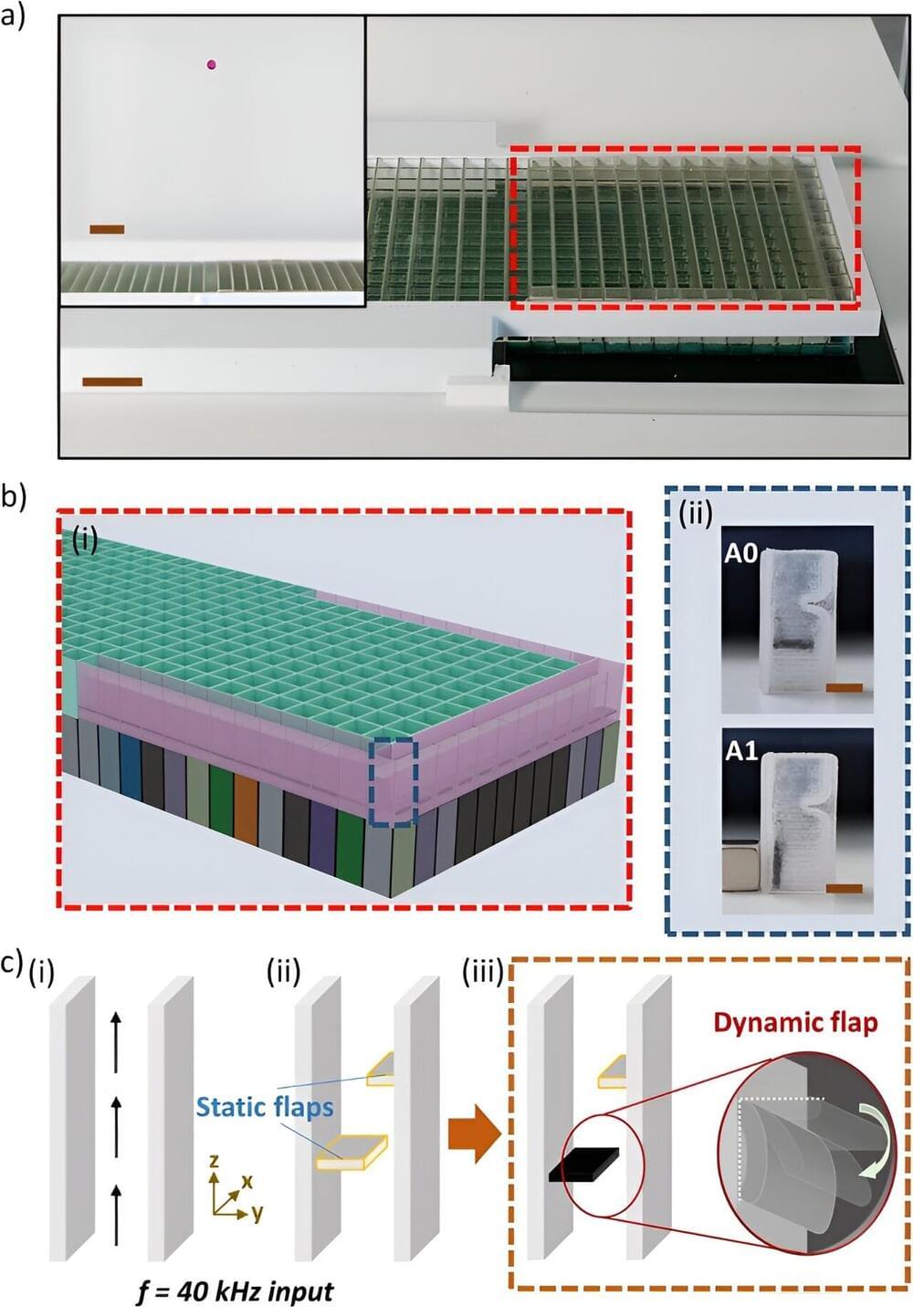
Space coiling acoustic metamaterials are static and require manual reconfiguration for sound-field modulation. In a new report published in Communications Materials, Christabel Choi, and a team of scientists in computer science and engineering in the U.K., and Italy, developed an approach for active reconfiguration with standalone dynamics to space-coil unit cells known as dynamic meta-bricks.
The meta-bricks housed an actuatable, magnetorheological, elastomeric flap, to function like a switch and to directly regulate the transmitted ultrasound. The scientists showed the synergy between active and passive reconfigurability to develop multifunctional metamaterials with additional degrees of freedom, for design and implementation.