There are a lot of firsts here.



Star Bound is a book for anyone who wants to learn about the American space program but isn’t sure where to start. First and foremost, it’s a history—short, sweet, and straightforward. From rocketry pioneer Robert Goddard’s primitive flight tests in 1926 through the creation of NASA, from our first steps on the moon to construction of the International Space Station and planning a trip to Mars, readers will meet the people and projects that have put the United States at the forefront of space exploration. Along the way, they’ll learn:
• How the United States beat the Soviets to the moon.
• Why astronauts float in space (Hint: It’s not for lack of gravity!)

Mars was once a very wet planet, as is evident in its surface geological features. Scientists know that over the last 3 billion years, at least some water went deep underground, but what happened to the rest? Now, NASA’s Hubble Space Telescope and MAVEN (Mars Atmosphere and Volatile Evolution) missions are helping unlock that mystery.
“There are only two places water can go. It can freeze into the ground, or the water molecule can break into atoms, and the atoms can escape from the top of the atmosphere into space,” explained study leader John Clarke of the Center for Space Physics at Boston University in Massachusetts. “To understand how much water there was and what happened to it, we need to understand how the atoms escape into space.”
Clarke and his team combined data from Hubble and MAVEN to measure the number and current escape rate of the hydrogen atoms escaping into space. This information allowed them to extrapolate the escape rate backwards through time to understand the history of water on the red planet.

It’s 7 billion years ago, and the universe’s heyday of star formation is beginning to slow. What might our Milky Way galaxy have looked like at that time? Astronomers using NASA’s James Webb Space Telescope have found clues in the form of a cosmic question mark, the result of a rare alignment across light-years of space.
“We know of only three or four occurrences of similar gravitational lens configurations in the observable universe, which makes this find exciting, as it demonstrates the power of Webb and suggests maybe now we will find more of these,” said astronomer Guillaume Desprez of Saint Mary’s University in Halifax, Nova Scotia, a member of the team presenting the Webb results.
While this region has been observed previously with NASA’s Hubble Space Telescope, the dusty red galaxy that forms the intriguing question-mark shape only came into view with Webb. This is a result of the wavelengths of light that Hubble detects getting trapped in cosmic dust, while longer wavelengths of infrared light are able to pass through and be detected by Webb’s instruments.

WASHINGTON (AP) — Earth’s moon will soon have some company — a “mini moon.”
The mini moon is actually an asteroid about the size of a school bus at 33 feet (10 meters). When it whizzes by Earth on Sunday, it will be temporarily trapped by our planet’s gravity and orbit the globe — but only for about two months.
The space rock — 2024 PT5 — was first spotted in August by astronomers at Complutense University of Madrid using a powerful telescope located in Sutherland, South Africa.

The study of computational biology is essential to understanding this transition. By exploring how life processes information, we gain insights into the nature of consciousness and intelligence itself. Computational models are key to revealing how systems organize, adapt, and evolve toward greater complexity and self-awareness. This progression suggests a future where intelligence is no longer bound by biological limitations but extends into the realm of artificial systems, creating a symbiotic relationship between humans and machines.
Ultimately, NOOGENESIS challenges traditional scientific paradigms by framing the universe as an informational “self-simulating” entity, where consciousness plays a central role in its evolutionary processes. The origins of life, the evolution of intelligence, and the potential for a post-Singularity future are all part of this grand narrative. By embracing this view, we can cultivate a more comprehensive understanding of the universe and our place within it—one that recognizes the fundamental role of consciousness in shaping reality and guiding evolution toward the apotheosis of Omega Singularity, the final convergence of intelligence and complexity.
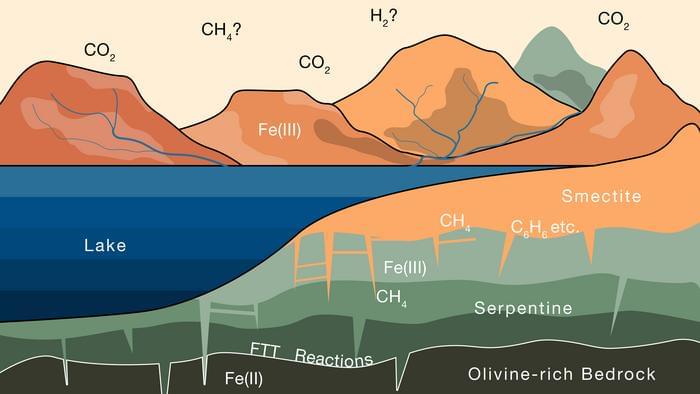
Billions of years ago, Mars is hypothesized to have been a much warmer and wetter planet featuring active volcanoes and vast liquid water oceans. However, something happened that caused the Red Planet to become the cold and dry world we see and explore today, but where did its atmosphere go? This is what a recent study published in Science Advances hopes to address as a team of researchers from the Massachusetts Institute of Technology (MIT) investigated how the large amounts of carbon that once existed in Mars’ atmosphere could now exist in the clay across the planet’s surface. This study holds the potential to help scientists better understand the formation and evolution of Mars and what that means in the search for life on the Red Planet, and beyond Earth.
For the study, the researchers calculated the amount of carbon storage within clays that potentially existed during what’s known as the Noachian Period on Mars, or between approximately 3.6 to 4 billion years ago. Their hypothesis is that when liquid water existed on the Red Planet, this water could have seeped its way into rocks, resulting in carbon dioxide being removed from the atmosphere and being converted into methane. In the end, the researchers calculated that the clays on Mars could potentially be housing up to 1.7 bar of carbon dioxide, or just over one standard atmosphere’s worth of carbon dioxide and approximately 80 percent of Mars’ ancient atmosphere.
“Based on our findings on Earth, we show that similar processes likely operated on Mars, and that copious amounts of atmospheric CO2 could have transformed to methane and been sequestered in clays,” said Dr. Oliver Jagoutz, who is a professor of geology in MIT’s Department of Earth, Atmospheric and Planetary Sciences (EAPS) and the sole co-author on the study. “This methane could still be present and maybe even used as an energy source on Mars in the future.”
A new study has mapped out the gravitational basins of attraction in the local universe, offering fresh insights into the large-scale cosmic structures that shape the movement of galaxies. The study has been published in Nature Astronomy.
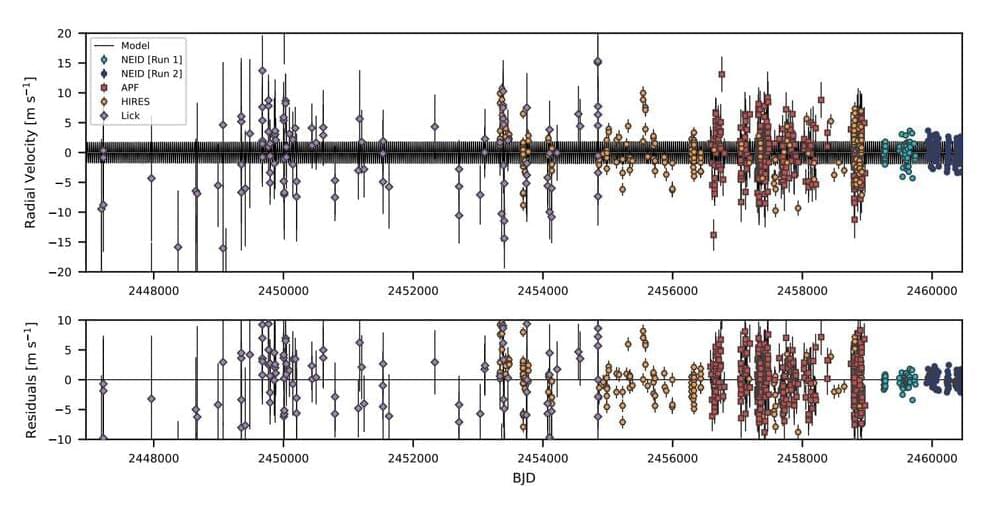
An international team of astronomers reports the discovery of a new extrasolar world orbiting a nearby star known as HD 86728. This is the first exoplanet detection made as part of the NEID Earth Twin Survey (NETS). The finding was detailed in a research paper published September 18 on the pre-print server arXiv.