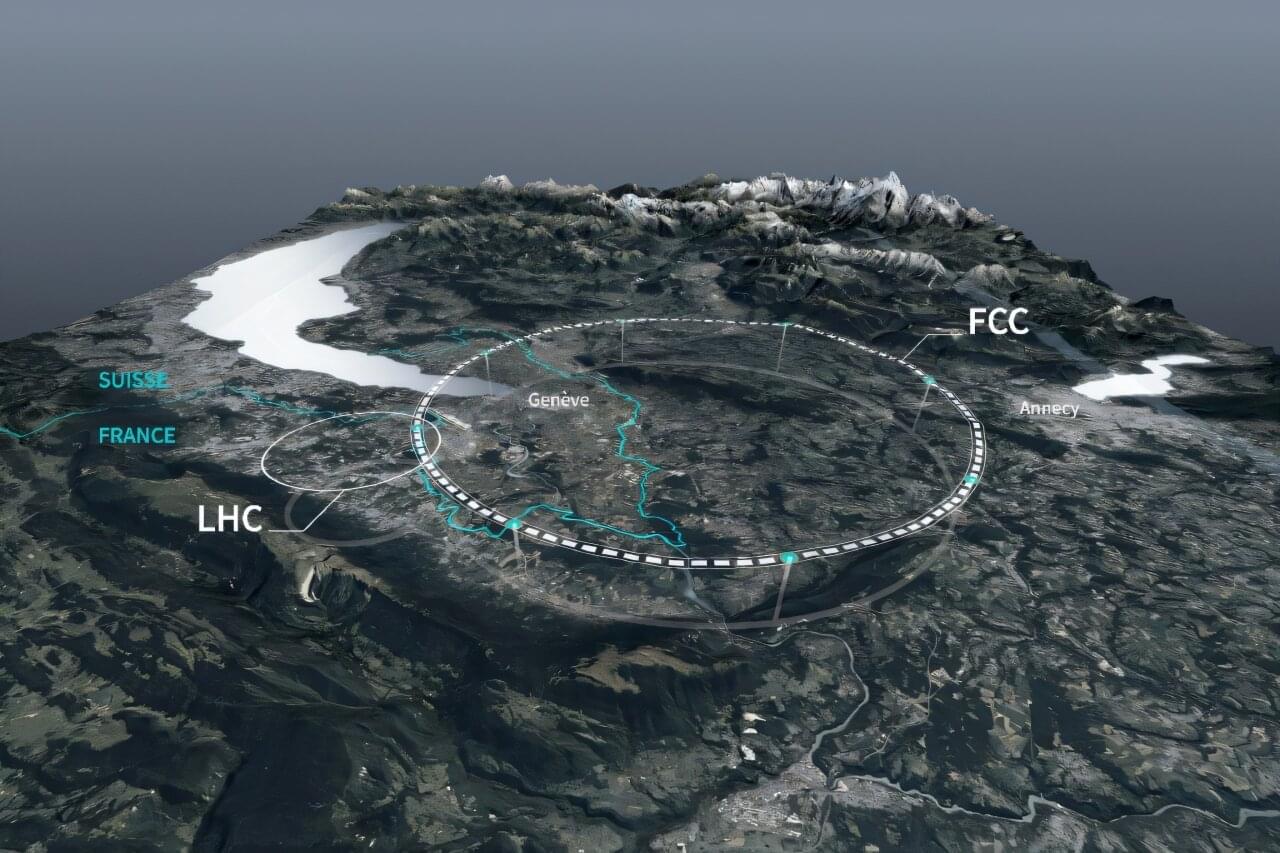
Europe’s physics lab CERN on Thursday said private donors had pledged $1 billion toward the construction of a new particle accelerator that would be by far the world’s biggest.
In a first, private individuals and philanthropic foundations have backed a flagship research project at CERN, the European Organization for Nuclear Research, which seeks to unravel what the universe is made of and how it works.
The donors include the Breakthrough Prize Foundation of billionaire Silicon Valley investor Yuri Milner; the Eric and Wendy Schmidt Fund for Strategic Innovation of former Google chief executive Eric Schmidt; plus Italian Agnelli family heir John Elkann, and French telecoms tycoon Xavier Niel.