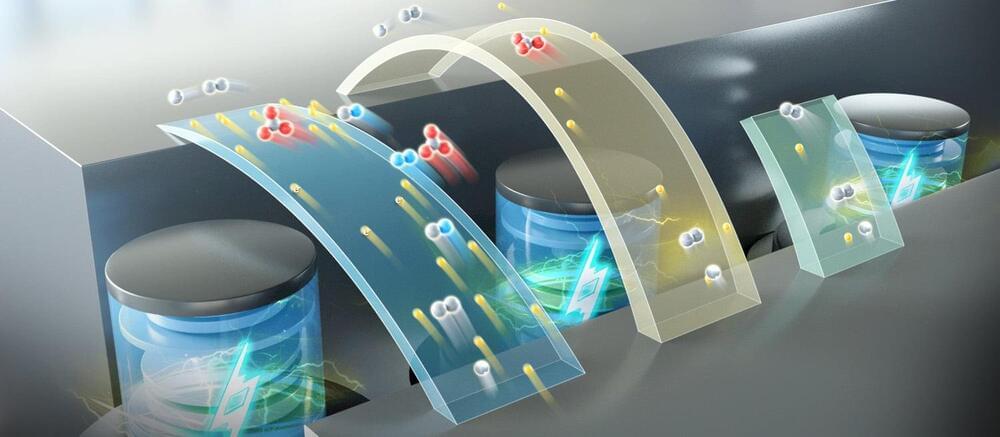
Traditional lithium-ion batteries, while offering high energy density, have compromised safety because they use flammable organic electrolytes.
Aqueous batteries use water as the solvent for electrolytes, significantly enhancing the safety of the batteries. However, due to the limited solubility of the electrolyte and low battery voltage, aqueous batteries typically have a lower energy density. This means that the amount of electricity stored per unit volume of aqueous battery is relatively low.
In a new study published in Nature Energy, a research group led by Prof. Li Xianfeng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. Fu Qiang’s group also from DICP, developed a multi-electron transfer cathode based on bromine and iodine, realizing a specific capacity of more than 840 Ah/L, and achieving an energy density of up to 1,200 Wh/L based on catholyte in full battery testing.