In biological imaging, researchers aim to achieve 3D, high-speed, and high-resolution, with low photobleaching and phototoxicity. The light-sheet fluorescence microscope (LSFM) helps meet that aim. Based on a unique excitation and detection scheme, the LSFM can image live specimens with high spatiotemporal resolution and low photobleaching. It has shown great potential for 3D imaging of biological samples.
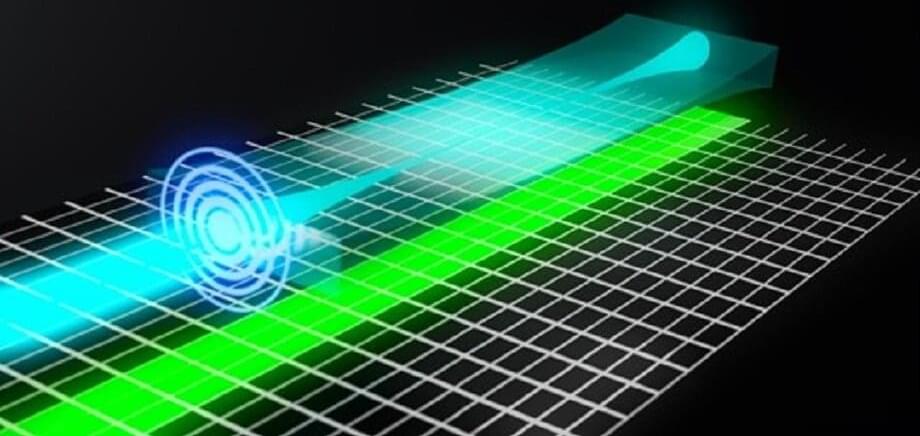
The principle of LSFM technology is to illuminate the sample with a thin light-sheet and then collect the emitted fluorescence along the axis perpendicular to the transmission of the light-sheet. Therefore, only fluorophores close to the focal plane are excited and detected. Using a thinner light-sheet improves the axial resolution, while a longer light-sheet improves the field of view (FoV) and imaging speed. Tradeoffs are required, as it is difficult to generate a thin, uniform light-sheet.
Multiple light-sheets can be tiled to generate a virtual light-sheet with a higher aspect ratio. However, multiple beams also introduce sidelobes, decreasing the axial resolution and optical sectioning. Axially swept light-sheet microscopy (ASLM) uses a slit to reject the sidelobes. It uses the rolling shutter of the sCMOS, which naturally serves as a slit, to synchronize beam scanning. ASLM can image an arbitrarily large FoV with optimal axial resolution. However, the fluorescence signal outside the rolling shutter will be rejected, so a larger FoV comes at the price of lower photon efficiency.