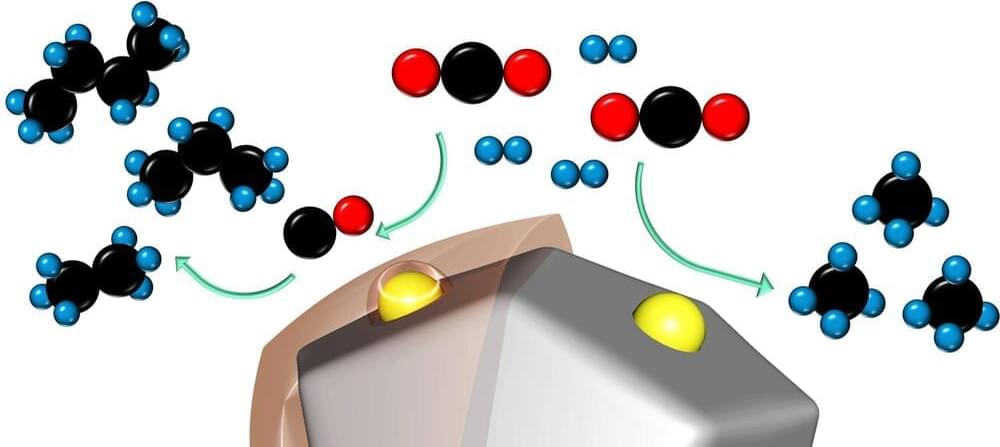
Engineers working to reverse the proliferation of greenhouse gases know that in addition to reducing carbon dioxide emissions we will also need to remove carbon dioxide from power plant fumes or from the skies. But, what do we do with all that captured carbon? Matteo Cargnello, a chemical engineer at Stanford University, is working to turn it into other useful chemicals, such as propane, butane or other hydrocarbon fuels that are made up of long chains of carbon and hydrogen.
“We can create gasoline, basically,” said Cargnello, who is an assistant professor of chemical engineering. “To capture as much carbon as possible, you want the longest chain hydrocarbons. Chains with eight to 12 carbon atoms would be the ideal.”
A new catalyst, invented by Cargnello and colleagues, moves toward this goal by increasing the production of long-chain hydrocarbons in chemical reactions. It produced 1,000 times more butane—the longest hydrocarbon it could produce under its maximum pressure—than the standard catalyst given the same amounts of carbon dioxide, hydrogen, catalyst, pressure, heat and time. The new catalyst is composed of the element ruthenium—a rare transition metal belonging to the platinum group—coated in a thin layer of plastic. Like any catalyst, this invention speeds up chemical reactions without getting used up in the process. Ruthenium also has the advantage of being less expensive than other high-quality catalysts, like palladium and platinum.