The groups also explained why in previous studies by other scientists, the chromatin appeared to fill the cell nuclei. “When scientists plate cells on a glass slide in order to study them under a microscope, they change their volume and physically flatten them. This may perturb some of the forces governing chromatin arrangement and reduce the distance between the upper part of the nucleus to its base,” Safran explains.
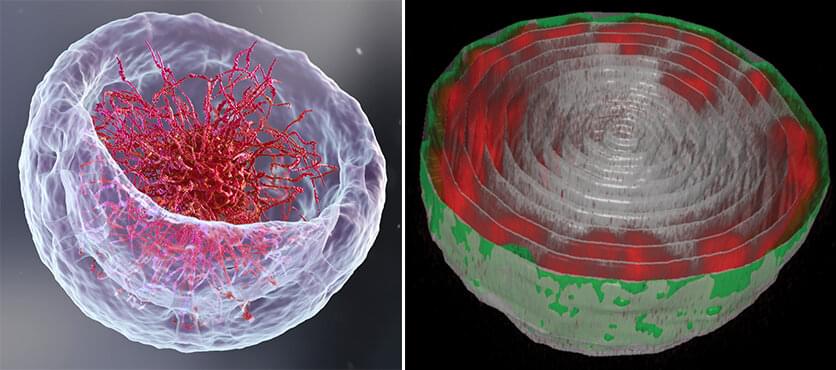
If you open a biology textbook and run through the images depicting how DNA is organized in the cell’s nucleus, chances are you’ll start feeling hungry; the chains of DNA would seem like a bowl of ramen: long strings floating in liquid. However, according to two new studies—one experimental and the other theoretical—that are the outcome of the collaboration between the groups of Prof. Talila Volk of the Molecular Genetics Department and Prof. Sam Safran of the Chemical and Biological Physics Department at the Weizmann Institute of Science, this image should be reconsidered. Clarifying it is essential since DNA’s spatial arrangement in the nucleus can affect the expression of genes contained within the DNA molecule, and hence the proteins found in the cell.
This story began when Volk was studying how mechanical forces influence cell nuclei in the muscle and found evidence that muscle contractions had an immediate effect on gene expression patterns. “We couldn’t explore this further because existing methods relied on imaging of chemically preserved cells, so they failed to capture what happens in the cell nuclei of an actual working muscle,” she says.
To address this issue, Dr. Dana Lorber, a research associate in Volk’s group, led the design of a device that makes it possible to study muscle nuclei in live fruit fly larvae. The device holds the tiny, translucent larva within a groove that allows it to contract and relax its muscles but keeps its movement constrained so that it can be scanned by a fluorescence microscope. Using the device, the researchers obtained images of the internal, linearly-organized complexes of DNA and its proteins (known as chromatin), surrounded by the membrane of the muscle nuclei.