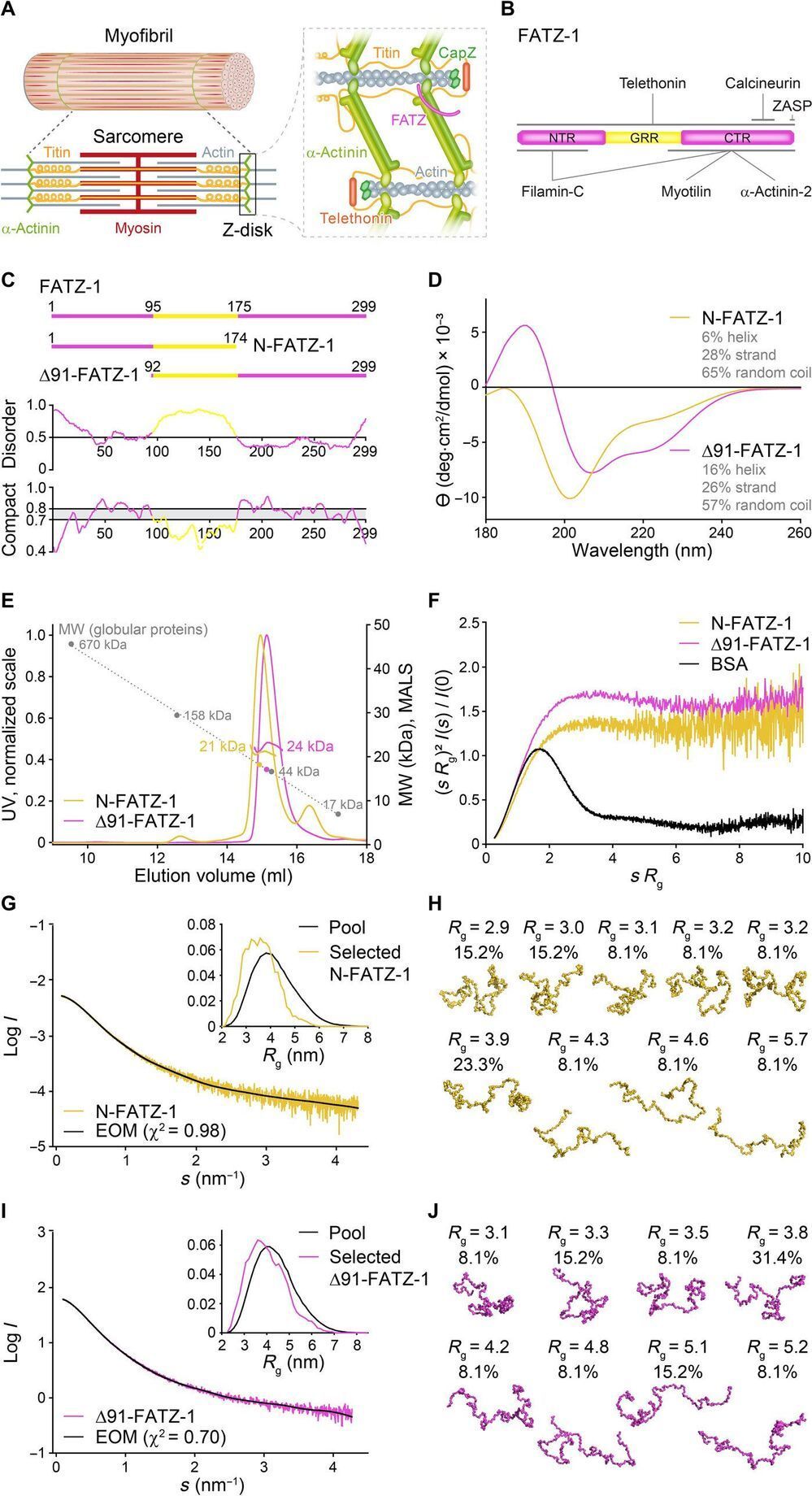
Alpha-actinin can cross-link actin filaments and anchor them to the Z-disk in sarcomeres. Sarcomeres are a structural unit of myofibril in striated muscle. The FATZ (filamin, α-actinin-and telethonin-binding protein of the Z-disk) protein can interact with α-actinin and other core Z-disk proteins that contribute to myofibril assembly and maintenance. In a new report now on Science Advances, Antonio Sponga and an international research team in Austria, Germany, Russia, Poland and the U.K. detailed the first structure and cellular validation of the α-actinin-2 complex with a Z-disk partner, FATZ-1, to form a conformal ensemble. The FATZ-1 formed a tight fuzzy complex with α-actinin-2 with a proposed interaction mechanism via molecular recognition elements and secondary binding sites. The obtained integrative model revealed a polar architecture of the complex in combination with the FATZ-1 multivalent scaffold function to organize interaction partners and stabilize.
Sarcomere
The contracting muscles can regulate voluntary animal movement and involuntary heart beating, and sarcomeres are the basic contractile units of striated muscle cells. They are composed of arrays of thin (actin) and thick (myosin) filaments that slide past each other during contraction. The Z-disk can form the boundary between adjacent sarcomeres, where anti-parallel actin filaments are anchored. A suitably stable anchoring structure must be generated by the interaction between myosin and actin. The Z-disk can fulfill this role by acting as a mechanical hub and a signaling platform to allow the transmission of tension during contraction and the duration and transmission of information of biomechanical stress. As a result, any mutations that disrupt the Z-disk architecture and function could risk causing skeletal and cardiac dysfunction.