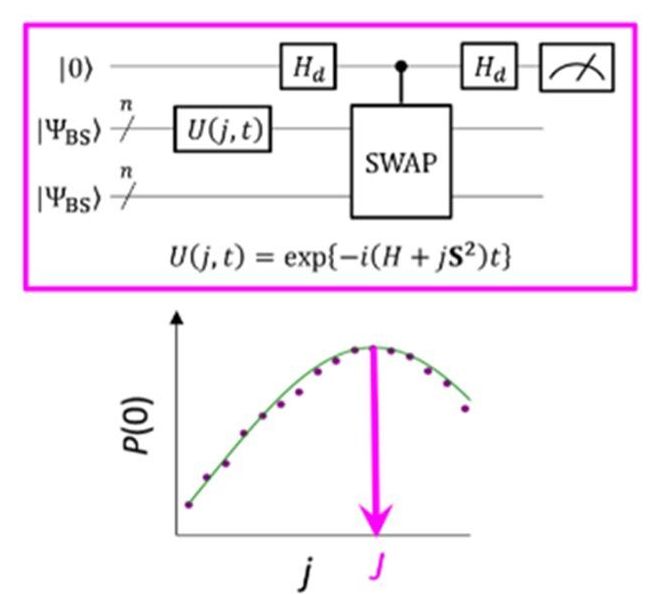
Researchers at Osaka City University use quantum superposition states and Bayesian inference to create a quantum algorithm, easily executable on quantum computers, that accurately and directly calculates energy differences between the electronic ground and excited spin states of molecular systems in polynomial time.
Understanding how the natural world works enables us to mimic it for the benefit of humankind. Think of how much we rely on batteries. At the core is understanding molecular structures and the behavior of electrons within them. Calculating the energy differences between a molecule’s electronic ground and excited spin states helps us understand how to better use that molecule in a variety of chemical, biomedical and industrial applications. We have made much progress in molecules with closed-shell systems, in which electrons are paired up and stable. Open-shell systems, on the other hand, are less stable and their underlying electronic behavior is complex, and thus more difficult to understand. They have unpaired electrons in their ground state, which cause their energy to vary due to the intrinsic nature of electron spins, and makes measurements difficult, especially as the molecules increase in size and complexity.