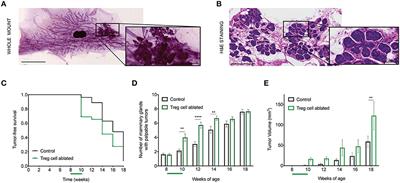
Ductal carcinoma in situ (DCIS) is a non-obligate precursor of breast cancer, and it only progresses to invasive breast cancer in around 40% of patients. While immune infiltrates have been observed in these early cancer lesions, their potential prognostic value is still unclear. Regulatory T (Treg) cells accumulate in advanced breast cancers, and predict poor outcome. We have shown before that ablation of Treg cells in established tumors leads to significant decrease in primary and metastatic tumor burden. In this work, we sought to investigate Treg cell function in the progression from non-invasive to invasive breast cancer lesions. To this end, we used the murine mammary tumor virus polyoma middle T (MMTV-PyMT) murine model of spontaneous, stage-wise breast carcinogenesis crossed to Foxp3DTR knock in mice, allowing Treg cell ablation by administration of diphtheria toxin. Transient targeting of Treg cells at the in situ carcinoma stage resulted in a significant increase in the number of tumor-bearing mammary glands and size of growing tumors compared with control mice. Whole mammary gland mounts and histological examination confirmed larger emergent tumor area in Treg cell-ablated mice, and revealed that these tumors were characterized by a more advanced tumor staging, with presence of early invasion, increased desmoplasia and collagen deposition. Furthermore, Treg cell ablation increased the percentage of cancer stem/progenitor cells in the mammary compartment. Interestingly, Treg cell ablation resulted in increased inflammatory cytokines IL-4 and IL-5 with a concomitant reduction in classically activated tumor associated macrophages. This TH2-biased immune regulatory mammary inflammation was consistent with the enhancement in tumor promotion that we observed. Overall, our study demonstrates that Treg cells oppose breast cancer progression at early stages, raising a cautionary note regarding the consideration of immune intervention targeted at boosting immune responses for DCIS.
While death from breast cancer has slowly declined in the past few years, mammographic screening has led to a dramatic increase in the detection of pre-invasive breast lesions in women (1–3). This paradoxical observation can be explained by the fact that only a low percentage of early breast disease progresses to invasive, metastatic carcinomas. Ductal carcinoma in situ (DCIS) is a heterogenous group of neoplastic lesions confined to the breast ducts, and can remain indolent for life in up to 60% of cases. Patients diagnosed with DCIS undergo breast-conserving therapy or mastectomy, frequently accompanied by radiotherapy and in some cases, hormonal therapy. Thus far, there are no reliable parameters to distinguish those cases that will progress, resulting in significant overtreatment.