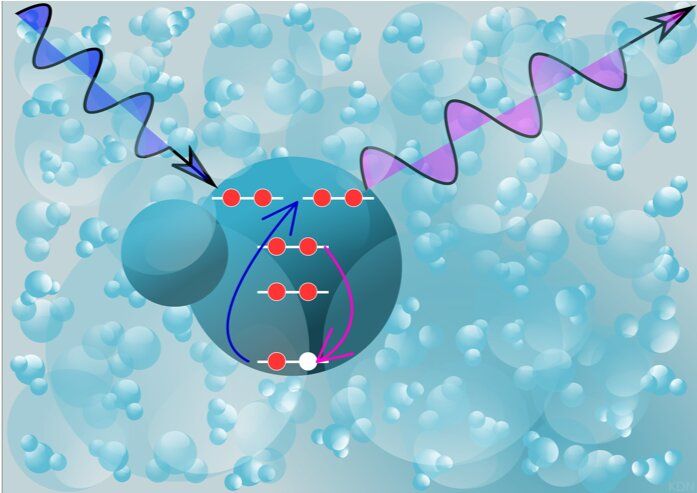
Free radicals—atoms and molecules with unpaired electrons—can wreak havoc on the body. They are like jilted paramours, destined to wander about in search of another electron, leaving broken cells, proteins and DNA in their wakes.
Hydroxyl radicals are the most chemically aggressive of the free radicals, surviving for only trillionths of a second. They form when water, the most abundant molecule in cells, is hit with radiation, causing it to lose an electron. In previous research, a team led by Linda Young, a scientist at the Department of Energy’s Argonne National Laboratory, observed the ultrafast birth of these free radicals, a process with great significance in fields such as sunlight-induced biological damage, environmental remediation, nuclear engineering, and space travel.
Now her team, including researchers from DOE’s SLAC National Accelerator Laboratory, has teased out a unique chemical fingerprint of the hydroxyl, which will help scientists track chemical reactions it instigates in complex biological environments. They published their results in Physical Review Letters in June.