Misfolding proteins and aggregates are a serious problem for a cell; a great range of research has been able to link poor protein ‘quality control’ with a whole range of diseases, perhaps most famously Alzheimer’s disease. Recent work also suggests that the ‘heat shock’ response, a mechanism that protects against misfolding and corrects badly made proteins, may also become impaired with aging. This gradual deterioration could turn out to be one of the most significant drivers of both aging and age-related disease.
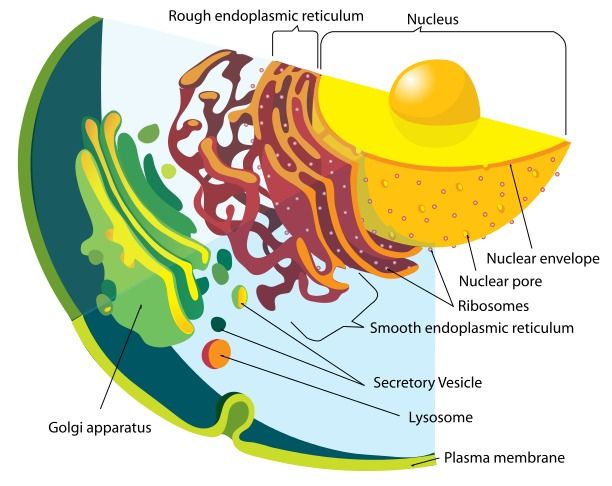
In research that support this theory, a recent paper provides evidence that the endoplasmic reticulum (ER), a cellular compartment which is responsible for creating and correctly forming protein structures, loses its oxidative power with age. This means that it loses the ability to form a type of bond called a disulphide bridge, a strong chemical bond which normally stabilises protein structures and holds them in particular shapes. The chemical environment within the ER was shown to change with age, disrupting the delicate equilibrium in the cell and leading to increased oxidative damage in other areas. Proteins moving through the ER on a production line often require disulphide linkages to mature correctly and stabilise their structure, but without this step they’re unable to do so and remain unstable.