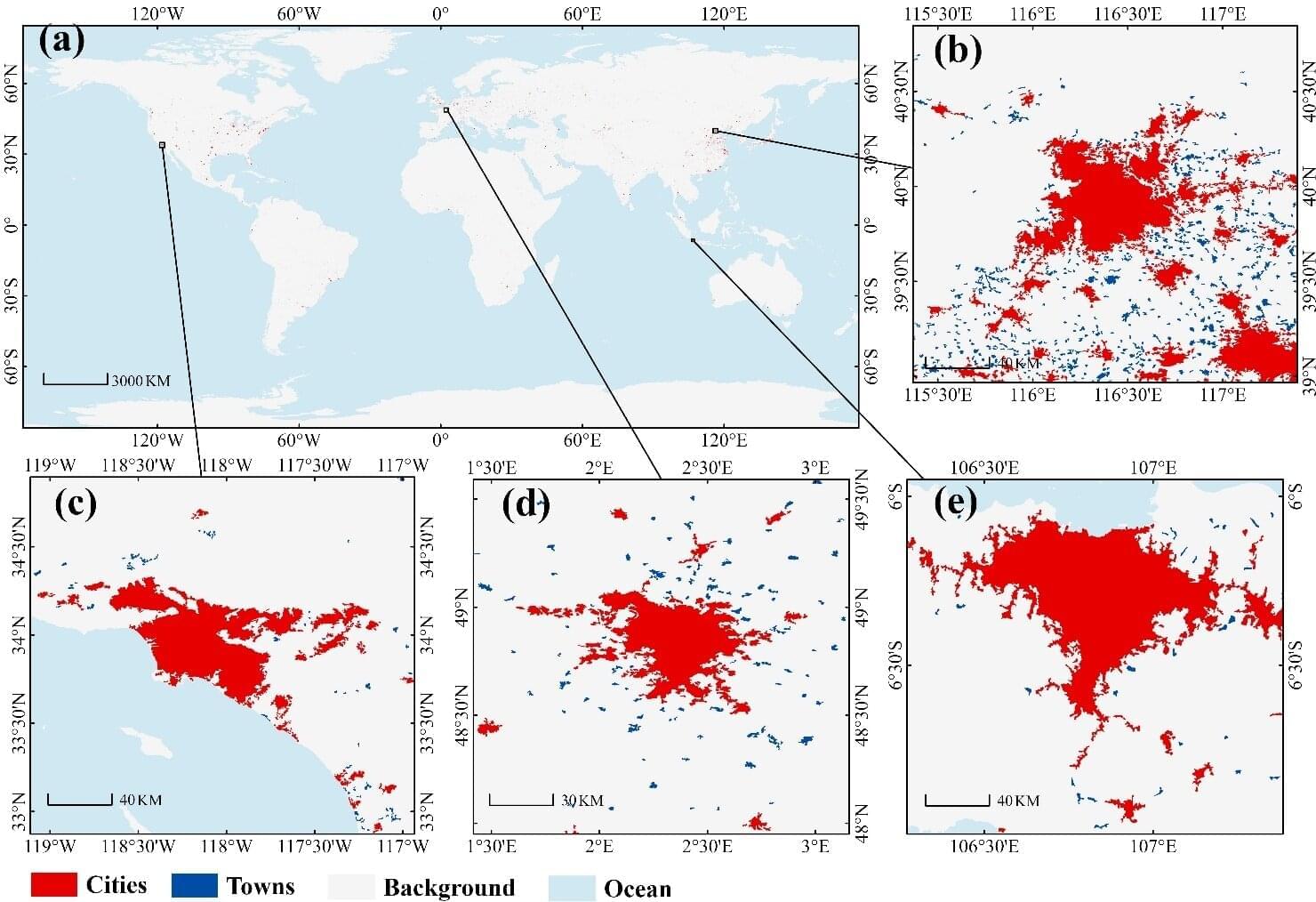
Thousands of glaciers will vanish each year in the coming decades, leaving only a fraction standing by the end of the century unless global warming is curbed, a study showed on Monday.
Government action on climate change could determine whether the world loses 2,000 or 4,000 glaciers annually by the middle of the century, according to the research.
A few degrees could be the difference between preserving almost half of the world’s glaciers in 2100—or fewer than 10%.