Japan researchers harness quantum entanglement to boost robot posture control.
Explore how researchers are using quantum computing to enhance the movement of robots, making them more efficient and smooth.

An incredible breakthrough brings quantum-scale precision sensing to living biological systems.
Quantum dots – semiconductor nanostructures that can emit single photons on demand – are considered among the most promising sources for photonic quantum computing.
However, every quantum dot is slightly different and may emit a slightly different color, according to a team at the University of Innsbruck, Austria, which has developed a technique to improve multi-photon state generation. The Innsbruck team states that, “the different forms of quantum dot means that, to produce multi-photon states we cannot use multiple quantum dots.”
Usually, researchers use a single quantum dot and multiplex the emission into different spatial and temporal modes, using a fast electro-optic modulator. But a contemporary technological challenge: faster electro-optic modulators are expensive and often require very customized engineering. To add to that, it may not be very efficient, which introduces unwanted losses in the system.
Nature Publishing: https://www.nature.com/articles/s41534-025-01083-0
Security wise: The team’s work combines years of research in quantum optics, semiconductor physics, and photonic engineering to open the door for next-generation quantum computers andunwanted losses in the system.
Communications. Here’s what you need to know. Securities IO: https://www.securities.io/passive-two-photon-quantum-dots-secure-communication
Researchers have developed QuantumShield-BC, a blockchain framework designed to resist attacks from quantum computers by integrating post-quantum cryptography (PQC) utilising algorithms such as Dilithium and SPHINCS+, quantum key distribution (QKD), and quantum Byzantine fault tolerance (Q-BFT) leveraging quantum random number generation (QRNG) for unbiased leader selection. The framework was tested on a controlled testbed with up to 100 nodes, demonstrating resistance to simulated quantum attacks and achieving fairness through QRNG-based consensus. An ablation study confirmed the contribution of each quantum component to overall security, although the QKD implementation was simulated and scalability to larger networks requires further investigation.
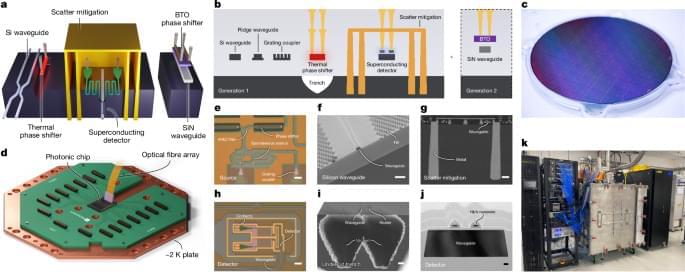
Quantum computers hold the potential of solving some optimization and data processing problems that cannot be tackled by classical computers. Many of the most promising quantum computing platforms developed so far are based on superconducting qubits, tiny circuits based on superconducting materials.

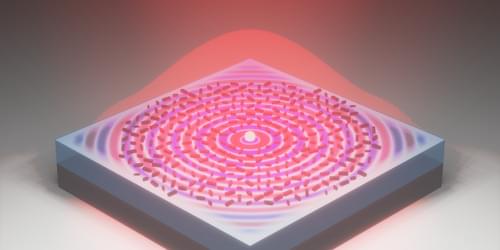
A 10-µm-wide microchip can generate light with any desired direction, polarization, and intensity, which will be handy for future quantum technologies.
Emerging technologies for quantum computing and cryptography require small components capable of emitting photons whose properties are precisely controlled. Researchers have been developing such components, and now a team has demonstrated a technique that provides control of direction, polarization, and intensity simultaneously [1]. Like previous experiments, the technique uses microscopic structures on a semiconductor surface to convert wave-like surface excitations to light waves. But the new demonstration uses shapes for these structures that allow more precise control over the outgoing light. The team expects the new technique to find wide use in efforts to build quantum technologies in miniature solid-state devices.
Solid-state miniaturization is one of the few realistic routes toward making quantum technologies practical, scalable, and easily manufacturable, says Fei Ding of the University of Southern Denmark. But there are not many good compact photon sources. “The technology really requires a compact and flexible solid-state photon source that gives us full control over how light is emitted—its direction, polarization, and spatial profile,” Ding says. “This is crucial for building scalable quantum and nanophotonic technologies, where single photons are used as the fundamental carriers of information.”