When the COVID-19 pandemic shut down experiments at the Department of Energy’s SLAC National Accelerator Laboratory early last year, Shambhu Ghimire’s research group was forced to find another way to study an intriguing research target: quantum materials known as topological insulators, or TIs, which conduct electric current on their surfaces but not through their interiors.
Denitsa Baykusheva, a Swiss National Science Foundation Fellow, had joined his group at the Stanford PULSE Institute two years earlier with the goal of finding a way to generate high harmonic generation, or HHG, in these materials as a tool for probing their behavior. In HHG, laser light shining through a material shifts to higher energies and higher frequencies, called harmonics, much like pressing on a guitar string produces higher notes. If this could be done in TIs, which are promising building blocks for technologies like spintronics, quantum sensing and quantum computing, it would give scientists a new tool for investigating these and other quantum materials.
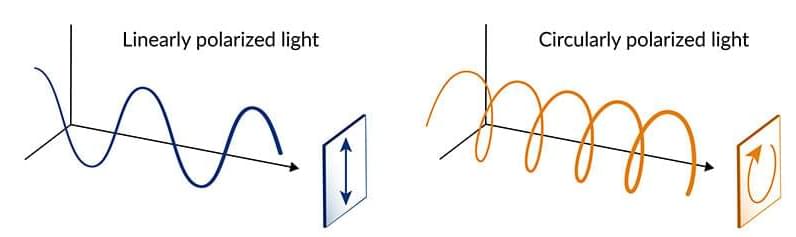
With the experiment shut down midway, she and her colleagues turned to theory and computer simulations to come up with a new recipe for generating HHG in topological insulators. The results suggested that circularly polarized light, which spirals along the direction of the laser beam, would produce clear, unique signals from both the conductive surfaces and the interior of the TI they were studying, bismuth selenide—and would in fact enhance the signal coming from the surfaces.