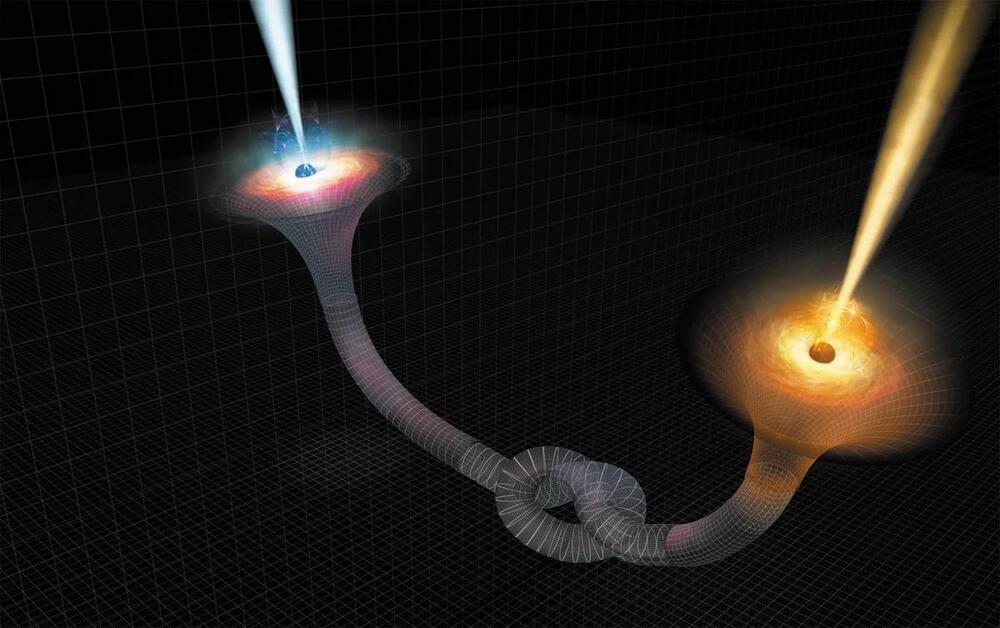
A team of physicists from Sofia University in Bulgaria has proposed a fascinating theory that wormholes, hypothetical tunnels linking different parts of the universe, could be hiding in plain sight. These wormholes may resemble black holes so closely that current technology cannot distinguish between the two, according to a new study reported by New Scientist.
Black holes have long been a source of mystery. They absorb everything, even light, leaving no trace of what falls into them. But where does the swallowed matter go? Some physicists have speculated that black holes might connect to “white holes,” which would spew out particles and radiation on the other end. Together, these phenomena could form a wormhole, or more specifically, an Einstein-Rosen bridge, connecting distant regions of space and time.