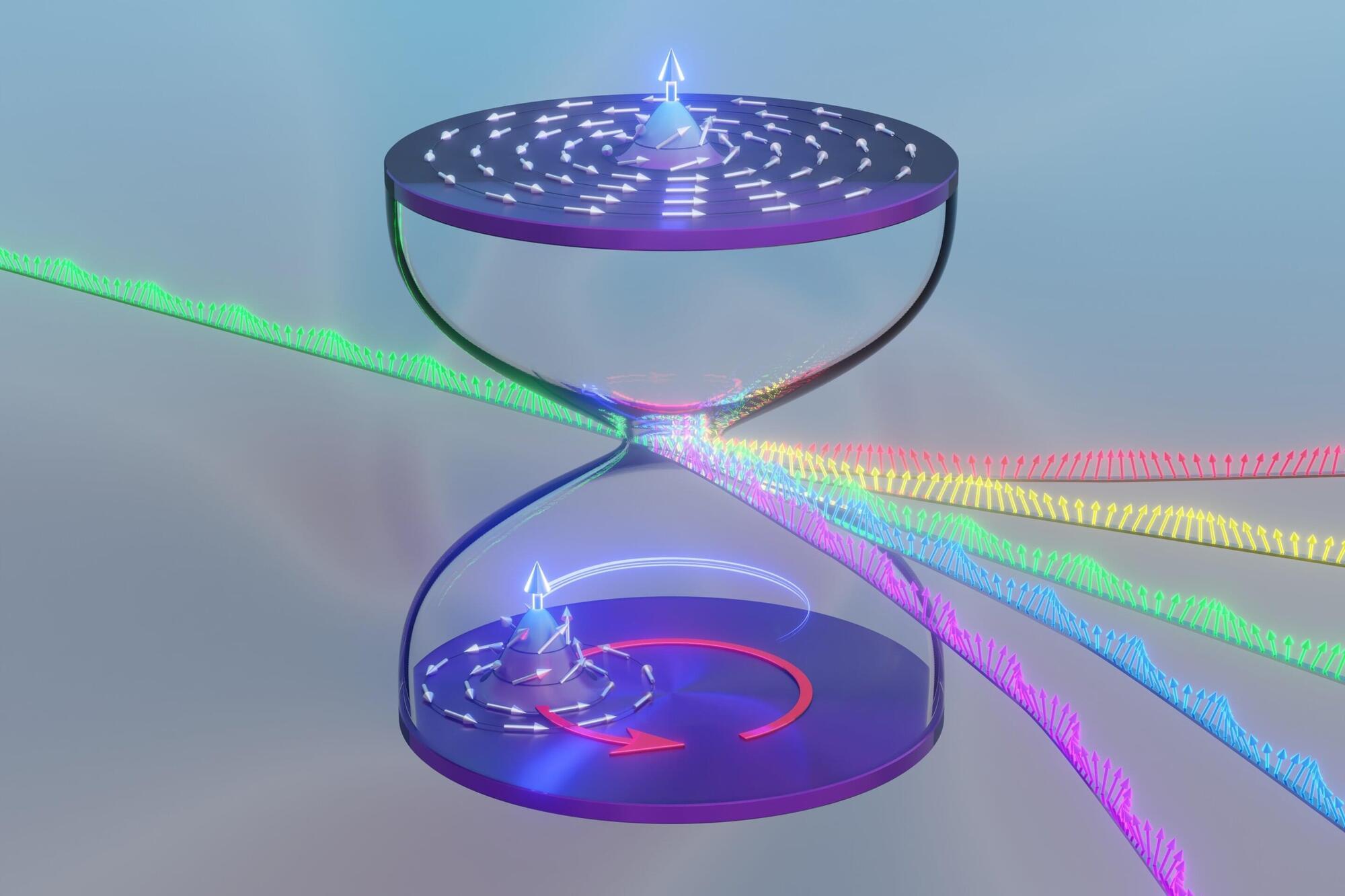
A collaboration between Stuart Parkin’s group at the Max Planck Institute of Microstructure Physics in Halle (Saale) and Claudia Felser’s group at the Max Planck Institute for Chemical Physics of Solids in Dresden has realized a fundamentally new way to control quantum particles in solids. Writing in Nature, the researchers report the experimental demonstration of a chiral fermionic valve—a device that spatially separates quantum particles of opposite chirality using quantum geometry alone, without magnetic fields or magnetic materials.
The work was driven by Anvesh Dixit, a Ph.D. student in Parkin’s group in Halle, and the first author of the study, who designed, fabricated, and measured the mesoscopic devices that made the discovery possible.
“This project was only possible because we could combine materials with exceptional topological quality and transport experiments at the mesoscopic quantum limit,” says Anvesh Dixit. “Seeing chiral fermions separate and interfere purely due to quantum geometry is truly exciting.”