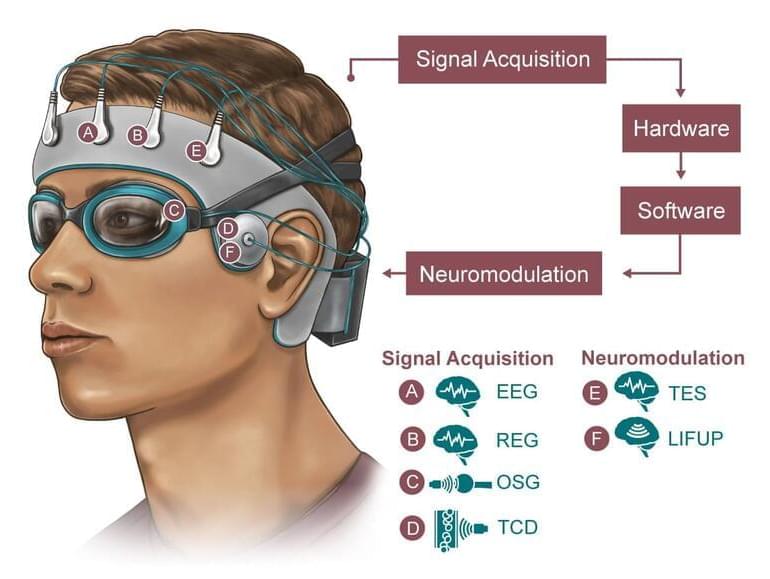
Although the idea of having a small device implanted in our skulls might sound terrifying to some, deep brain stimulation has had a successful past in other brain disorders such as Parkinson’s disease and epilepsy.
Depression can be a frighteningly relentless condition. Luckily, researchers around the world are constantly working on new treatment options, such as a newly designed brain implant for resistant depression.
Altogether, up to a third of people with depression don’t respond or become resistant to treatment. No medication or therapy type seems to help. For those with such treatment-resistant depression, the future can look especially bleak.
This is what happened to Sarah, a 36-year-old woman who’s had severe and treatment-resistant depression since she was a child. But a new proof-of-concept intervention has provided significant relief for Sarah, and could offer hope for many like her. The only catch? It requires a custom-designed ‘brain pacemaker’ for each person.