Category: neuroscience – Page 857

Neurons Are Fickle. Electric Fields Are More Reliable for Information
Summary: Electric fields may represent information held in working memory, allowing the brain to overcome representational drift.
Source: MIT
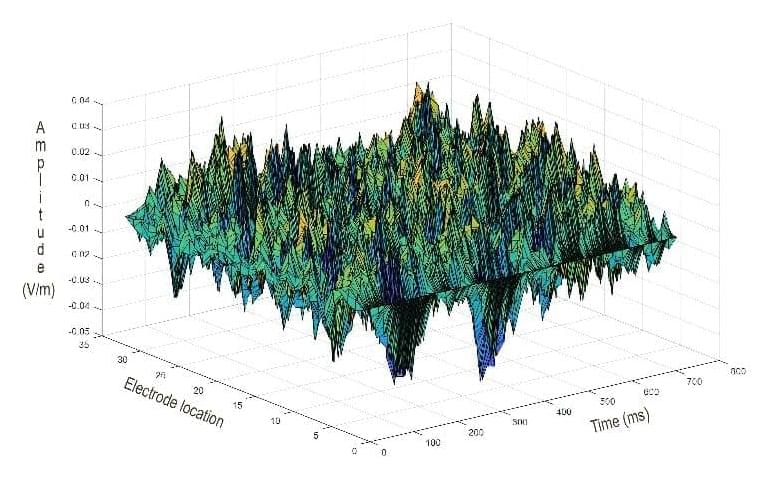
A new study suggests that electric fields may represent information held in working memory, allowing the brain to overcome “representational drift,” or the inconsistent participation of individual neurons.
Open-Access Dataset of Macaque Brain Published
Researchers have released a new open-access data set recorded from the visual cortex of macaque monkeys during rest state.
Summary: Researchers have released a new open-access data set recorded from the visual cortex of macaque monkeys during resting state.
Source: KNAW
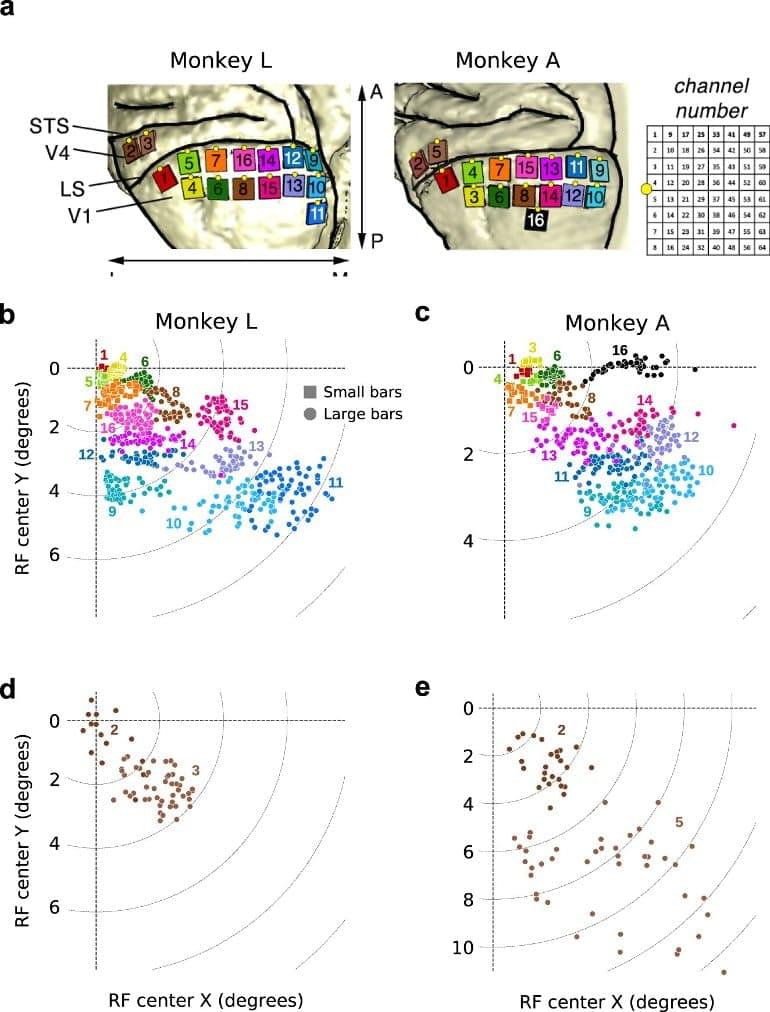
Researchers from the Netherlands Institute for Neuroscience (NIN) have published a dataset that was recorded from the visual cortex of monkeys during the resting state.
The dataset can be shared with other researchers for analysis, used as teaching material and/or serve as a template for future publications of large electrophysiology datasets, therefore less monkeys are needed for research.

New algorithm could help enable next-generation deep brain stimulation devices
Now, a new algorithm developed by Brown University bioengineers could be an important step toward such adaptive DBS. The algorithm removes a key hurdle that makes it difficult for DBS systems to sense brain signals while simultaneously delivering stimulation.
“We know that there are electrical signals in the brain associated with disease states, and we’d like to be able to record those signals and use them to adjust neuromodulation therapy automatically,” said David Borton, an assistant professor of biomedical engineering at Brown and corresponding author of a study describing the algorithm. “The problem is that stimulation creates electrical artifacts that corrupt the signals we’re trying to record. So we’ve developed a means of identifying and removing those artifacts, so all that’s left is the signal of interest from the brain.”

New discoveries of deep brain stimulation put it on par with therapeutics
Despite having remarkable utility in treating movement disorders such as Parkinson’s disease, deep brain stimulation (DBS) has confounded researchers, with a general lack of understanding of why it works at some frequencies and does not at others. Now a University of Houston biomedical engineer is presenting evidence in Nature Communications Biology that electrical stimulation of the brain at higher frequencies (100Hz) induces resonating waveforms which can successfully recalibrate dysfunctional circuits causing movement symptoms.
“We investigated the modulations in local field potentials induced by electrical stimulation of the subthalamic nucleus (STN) at therapeutic and non-therapeutic frequencies in Parkinson’s disease patients undergoing DBS surgery. We find that therapeutic high-frequency stimulation (130−180 Hz) induces high-frequency oscillations (~300 Hz, HFO) similar to those observed with pharmacological treatment,” reports Nuri Ince, associate professor of biomedical engineering.
For the past couple of decades, deep brain stimulation (DBS) has been the most important therapeutic advancement in the treatment of Parkinson’s disease, a progressive nervous system disorder that affects movement in 10 million people worldwide. In DBS, electrodes are surgically implanted in the deep brain and electrical pulses are delivered at certain rates to control tremors and other disabling motor signs associated with the disease.
How electrical stimulation reorganizes the brain
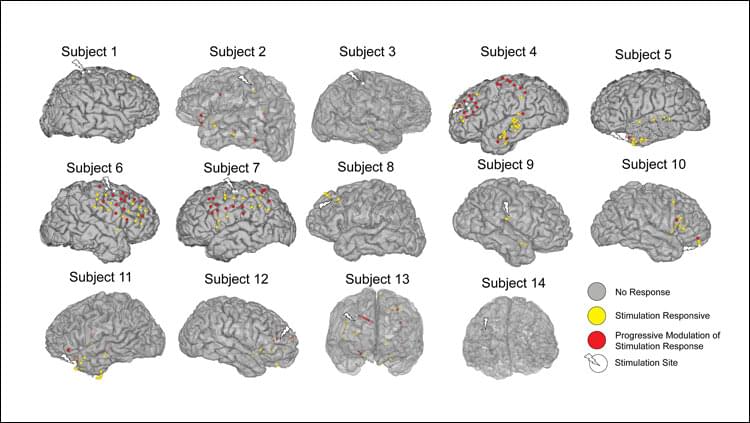
Recordings of neural activity during therapeutic stimulation can be used to predict subsequent changes in brain connectivity, according to a study of epilepsy patients published in JNeurosci. This approach could inform efforts to improve brain stimulation treatments for depression and other psychiatric disorders.
Corey Keller and colleagues delivered electrical stimulation from implanted electrodes in 14 patients while recording participants’ brain activity.
Repeated sets of stimulation resulted in progressive changes to the brain’s response to simulation, with stronger responses in brain regions connected to the stimulation site.
From drugs to brain surgery—the consciousness technology of the future
Our complicated emotional lives can often feel like a prison. Insecurities, depression and anxiety can all hold us back in life. But what if we could just eliminate the mental states that we don’t want? Or enhance the moods we do? There’s every reason to believe that this may be commonplace in the future. In fact, a lot of the technology that could achieve this already exists.
More than half of us will have experienced an extended period of sadness or low mood during our lives, and about a fifth will have been diagnosed with major depression, although these figures depend a lot on the culture in which you live. The fact that mood disorders are so common – and also so difficult to treat – means that research into the future of mood modulation is constantly evolving.
If you go to a doctor in the UK with suspected depression today, you will start on a pathway of care including “talking cures” such as cognitive behavioural therapy, or drug treatments including serotonin re-uptake inhibitors like Prozac. People who do not respond to these treatments may progress to heavier regimes or combinations of drug treatments. Since most psychoactive drug treatments are associated with side effects, there is pressure to develop new treatment options that are better tolerated by most people.
Precise control of brain circuit alters mood
Stress-susceptible animals that behaved as if they were depressed or anxious were restored to relatively normal behavior by tweaking the system, according to a study appearing in the July 20 issue of Neuron.
“If you ‘turn the volume up’ on animals that hadn’t experienced stress, they start normal and then they have a problem,” said lead researcher Kafui Dzirasa, an assistant professor of psychiatry and behavioral sciences, and neurobiology. “But in the animals that had experienced stress and didn’t do well with it, you had to turn their volume up to get them back to normal. It looked like stress had turned the volume down.”

Is Seeing Believing? How Neural Oscillations Influence Our Conscious Experience
Summary: The perceptual accuracy of visual information and its subjective interpretation use separate neural mechanisms that can be manipulated independently of each other.
Source: University of Bologna
A research group from the University of Bologna discovered the first causal evidence of the double dissociation between what we see and what we believe we see: these two different mechanisms derive from the frequency and amplitude of alpha oscillations.

Effective new target for mood-boosting brain stimulation found
Researchers have found an effective target in the brain for electrical stimulation to improve mood in people suffering from depression. As reported in the journal Current Biology on November 29, stimulation of a brain region called the lateral orbitofrontal cortex (OFC) reliably produced acute improvement in mood in patients who suffered from depression at the start of the study.
Those effects were not seen in patients without mood symptoms, suggesting that the brain stimulation works to normalize activity in mood-related neural circuitry, the researchers say.
“Stimulation induced a pattern of activity in brain regions connected to OFC that was similar to patterns seen when patients naturally experienced positive mood states,” says Vikram Rao, of the University of California, San Francisco. “Our findings suggest that OFC is a promising new stimulation target for treatment of mood disorders.”