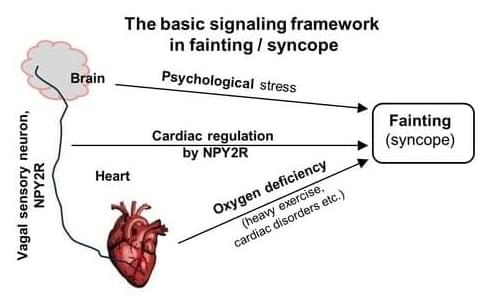
Observed and recorded in various forms since ancient times, ‘syncope’ is often popularly called ‘fainting’, such that the two terms are used synonymously.

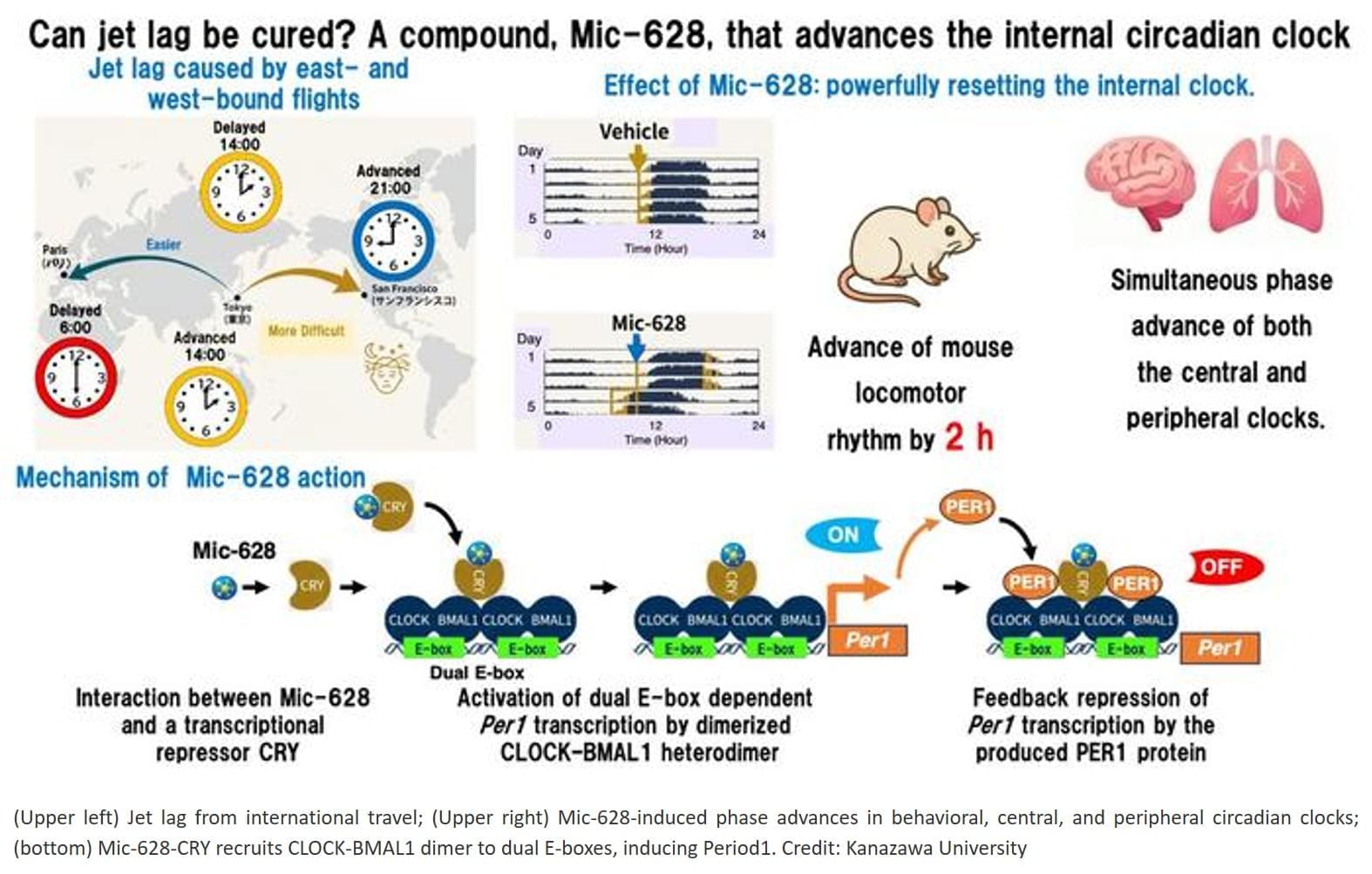
Adapting to eastward travel, such as west-to-east transmeridian flights, or to night-shift work requires advancing the internal clock, a process that normally takes longer and is physiologically harder than delaying it.
Existing methods, such as light therapy or melatonin, are heavily constrained by timing and often yield inconsistent results.
Mic-628’s consistent phase-advance effect, regardless of when it is administered, represents a new pharmacological strategy for resetting the circadian clock.
The researchers discovered that Mic-628 selectively induces the mammalian clock gene Per1.
Mic-628 works by binding to the repressor protein CRY1, promoting the formation of a CLOCK–BMAL1–CRY1–Mic-628 complex that activates Per1 transcription through a “dual E-box” DNA element.
As a result, both the central clock in the brain’s suprachiasmatic nucleus (SCN) and peripheral clocks in tissues such as the lungs were advanced—in tandem and independent of dosing time.
In a simulated jet lag mouse model (6-hour light-dark phase advance), a single oral dose of Mic-628 shortened re-entrainment time from seven days to four.

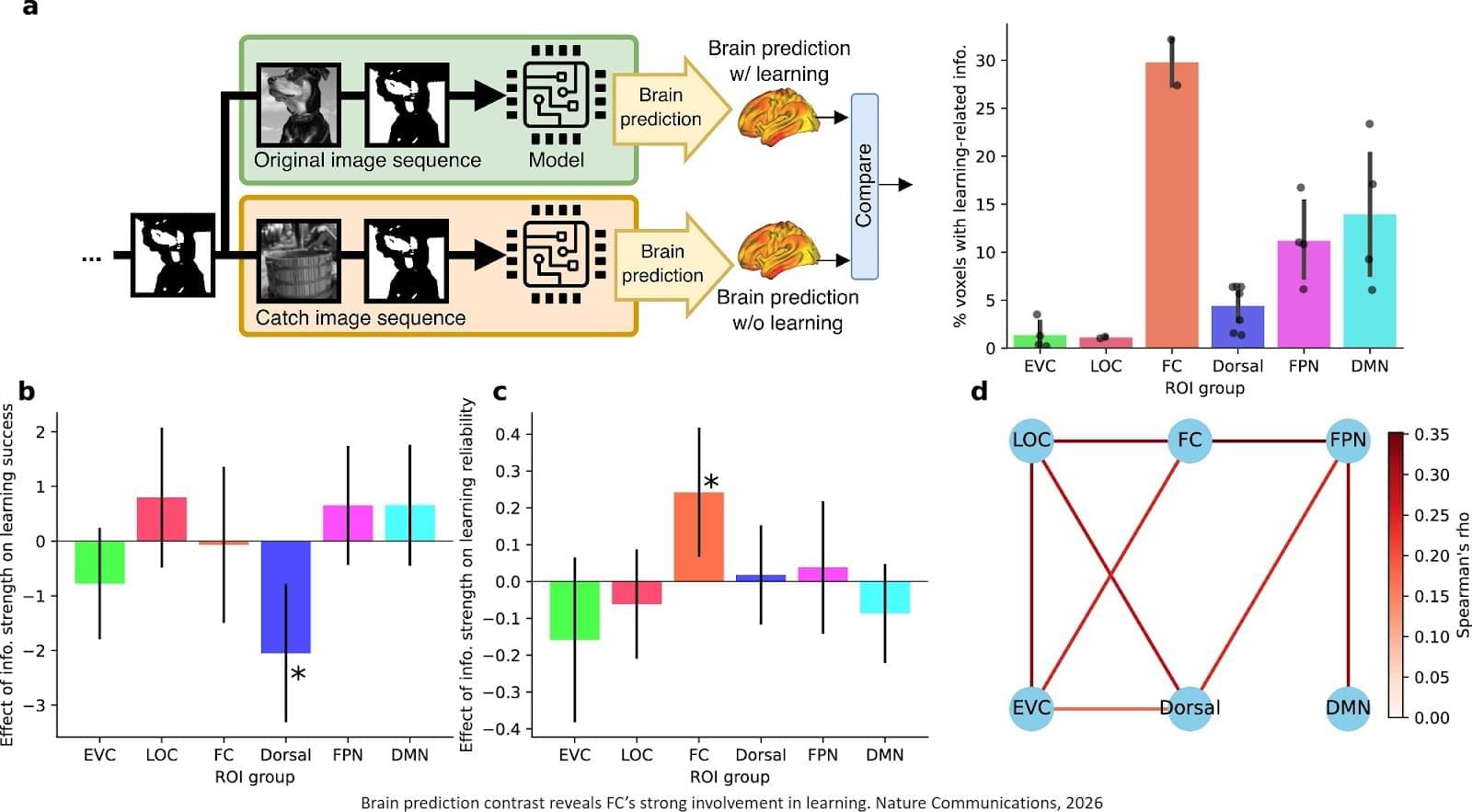
Despite decades of research, the mechanisms behind fast flashes of insight that change how a person perceives their world, termed “one-shot learning,” have remained unknown. A mysterious type of one-shot learning is perceptual learning, in which seeing something once dramatically alters our ability to recognize it again.
Now a new study, the researchers address the moments when we first recognize a blurry object, a primal ability that enabled our ancestors to avoid threats.
Published in Nature Communications, the new work pinpoints for the first time the brain region called the high-level visual cortex (HLVC) as the place where “priors” — images seen in the past and stored — are accessed to enable one-shot perceptual learning.
“Our work revealed, not just where priors are stored, but also the brain computations involved,” said co-senior study author.
Importantly, past studies had shown that patients with schizophrenia and Parkinson’s disease have abnormal one-shot learning, such that previously stored priors overwhelm what a person is presently looking at to generate hallucinations.
“This study yielded a directly testable theory on how priors act up during hallucinations, and we are now investigating the related brain mechanisms in patients with neurological disorders to reveal what goes wrong,” added the author.
The research team is also looking into likely connections between the brain mechanisms behind visual perception and the better-known type of “aha moment” when we comprehend a new idea. ScienceMission sciencenewshighlights.

A team led by researchers at Chalmers University of Technology, Sweden, has succeeded in identifying biomarkers for Parkinson’s disease in its earliest stages, before extensive brain damage has occurred. The biological processes leave measurable traces in the blood, but only for a limited period.
The discovery thus reveals a window of opportunity that could be crucial for future treatment, but also for early diagnosis via blood tests, which could begin to be tested in health care within five years.
Parkinson’s is an endemic disease with over 10 million people affected globally. As the world’s population grows older, this number is expected to more than double by 2050. At present, there is neither an effective cure nor an established screening method for detecting this chronic neurological disorder at an early stage before it has caused significant damage to the brain.

The human body is often described in parts—different limbs, systems, and organs—rather than something fully interconnected and whole. Yet many bodily processes interact in ways we may not always recognize. For example, researchers at the University of Missouri School of Medicine may have found a link between high blood pressure and an overactive nervous system.
The paper is published in the journal Cardiovascular Research.
High blood pressure, also called hypertension, is a common cardiovascular condition and a risk factor for multiple diseases and sudden health concerns like stroke or heart attack.

Exploring the Global Workspace Theory: Consciousness Unveiled. Join us as we unravel Bernard Baars’ groundbreaking Global Workspace Theory (GWT) and discover how our brain functions like a theater, spotlighting conscious experiences.
00:00:00 Introduction to Global Workspace Theory.
00:00:34 Global Broadcasting.
00:01:08 Evidence from Brain Imaging and EEG Studies.
00:01:35 Common Mechanism for Conscious Access.
00:02:21 Intracranial EEG Study by Gaillard and Colleagues.
00:02:56 Brain Activity Overdrive.
00:03:56 Neuronal Synchrony and Binocular Rivalry.
00:04:13 Rhythm of Awareness.
00:05:03 Dynamic Synchrony and Global Accessibility.
00:05:19 On-Air Sign for the Brain.
00:05:35 Long-Distance Coordination and Global Coherence.
00:06:04 The Brain Orchestra.
00:06:20 Global Workspace and Clinical Neurology.
00:06:49 Disrupting Global Connectivity.
00:07:22 Frontal Lobe Lesions and Blindsight.
00:08:01 Widespread Disconnection and Fading of Consciousness.
00:08:57 Recovery from Disorders of Consciousness.
00:09:16 Temporary Inactivation with TMS
00:10:12 Interrupting Broadcasting Hubs.
00:10:35 Restoring Connectivity and Consciousness.
00:11:26 Jump-Starting Thalamo-Cortical Circuits.
00:11:43 Re-Engaging the Global Workspace.
00:11:58 The Brain’s Internal News Feed.
00:12:31 Broadcasting and Subjective Experience.
Our consciousness is a fundamental aspect of our existence, says philosopher David Chalmers: “There’s nothing we know about more directly… but at the same time it’s the most mysterious phenomenon in the universe.” He shares some ways to think about the movie playing in our heads.

In a randomized clinical trial of women with prior HypertensivePregnancy, physician-optimized postpartum blood pressure self-management was associated with larger white matter brain volumes at 9 months compared with usual care.
Women with a history of preeclampsia receiving usual care had smaller volumes in subcortical regions (putamen, accumbens, pallidum) than those with gestational hypertension, differences that were not observed in the intervention group.
This randomized clinical trial indicates that a postpartum blood pressure management intervention after hypertensive disorders of pregnancy may be associated with favorable brain structure during the first year post partum. The intervention was linked to larger white matter volumes across women with hypertensive pregnancy (gestational hypertension and preeclampsia). In addition, women with a history of preeclampsia in the usual care arm showed smaller subcortical brain volumes at 6 to 9 months post partum than those with gestational hypertension; these differences were not evident among women in the intervention arm.
Both women with preeclampsia and gestational hypertension experience high blood pressure during pregnancy that frequently persists post partum.28 Lower white matter integrity has been reported from the peripartum period into later life.3,12,29 Hypertension-related white matter injury30,31 is associated with slower processing speed, executive dysfunction, and memory impairment.31 Although cognitive impact may not be obvious in the early postpartum period, white matter changes predict later cognitive decline and dementia,32 and converging longitudinal evidence suggests that reductions in white matter volume and integrity track cognitive decline, supporting the interpretation that better-preserved white matter is beneficial.33
Whether postpartum white matter changes are preventable or reversible had not been investigated. In this randomized clinical trial, a short-term blood pressure control intervention was associated with larger brain volumes several months later, when most participants were no longer taking antihypertensive medication. This is consistent with the postpartum period as a critical window for pregnancy-associated brain volume and blood pressure changes. Because baseline brain MRIs were not acquired, we cannot distinguish recovery of pregnancy-related changes from a slower postpregnancy decline relative to usual care.