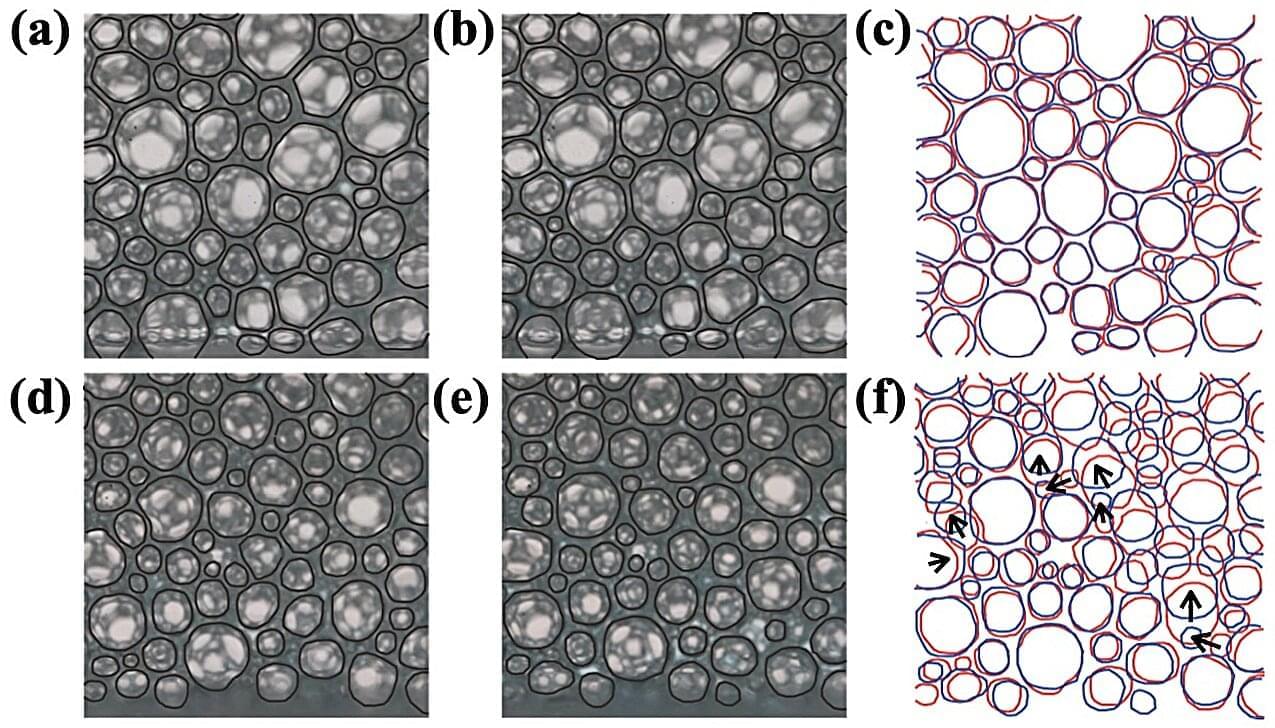
Researchers from Tokyo Metropolitan University have solved a long-standing mystery behind the drainage of liquid from foams. Standard physics models wildly overestimate the height of foams required for liquid to drain out the bottom. Through careful observation, the team found that the limits are set by the pressure required to rearrange bubbles, not simply push liquid through a static set of obstacles.
Their approach highlights the importance of dynamics to understanding soft materials. The study is published in the Journal of Colloid and Interface Science.
When you spray foam on a wall, you will often see droplets of liquid trailing out the bottom. That is because foams are a dense collection of bubbles connected by walls of liquid, forming a complex labyrinth of interconnected paths. It is possible for liquid to travel along these paths, either leaving the foam or sucking in liquid which is brought into contact with the foam.