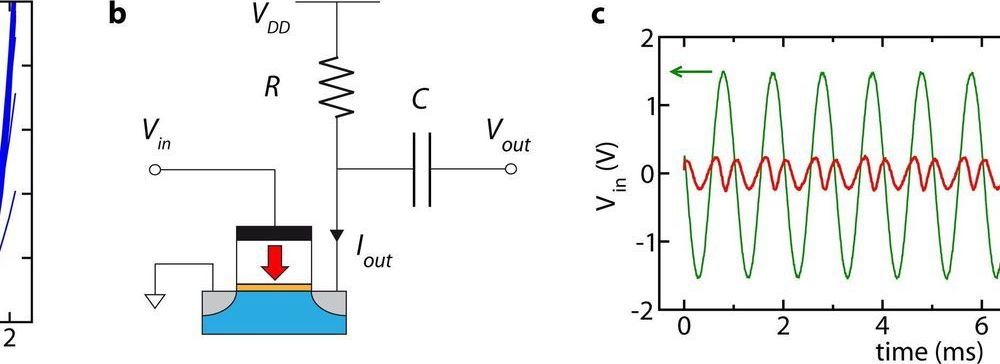
Frequency multipliers, circuits that can produce signals with multiple frequencies, are essential components for a number of technological tools, particularly wireless communications systems. Most existing multipliers, however, are built using filtering and amplification circuits that are bulky and rapidly drain a lot of power.
Researchers at NaMLab in Germany have recently devised a single ferroelectric field-effect transistor that can serve both as a full-wave rectifier and frequency multiplier. The device they developed, presented in a paper published in Nature Electronics, is fully reconfigurable and energy-efficient, as it can be used in isolation, not requiring any additional circuits.
“Our institute (NaMLab) has been doing research on ferroelectric hafnium oxide (HfO2) since this material’s ferroelectric properties were discovered in 2007,” Halid Mulaosmanovic, one of the researchers who carried out the study, told TechXplore. “An attractive electronic device that can be made using this material is a ferroelectric field-effect transistor (FeFET), which resembles conventional logic transistors, but has a ferroelectric layer in the gate stack.”