Graphene out garbage?
A recent breakthrough promises to make graphene out of garbage in a flash.

A major new milestone has just been achieved in the quest for superconductivity. For the first time, physicists have achieved the resistance-free flow of an electrical current at room temperature — a positively balmy 15 degrees Celsius (59 degrees Fahrenheit).
This has smashed the previous record of −23 degrees Celsius (−9.4 degrees Fahrenheit), and has brought the prospect of functional superconductivity a huge step forward.
“Because of the limits of low temperature, materials with such extraordinary properties have not quite transformed the world in the way that many might have imagined,” physicist Ranga Dias of the University of Rochester said in a press statement.

With NASA getting ready to land a spacecraft on the asteroid Bennu in just a few short days, the mysterious space rock is already revealing some of its secrets, including the presence of carbon-bearing materials.
Several studies were published on the matter in the journals Science and Science Advances, noting that carbon-bearing, organic material is “widespread” on the surface of the asteroid. This includes the area where NASA’s OSIRIS-REx spacecraft will take its first sample from, known as Nightingale, on Oct. 20.
“The abundance of carbon-bearing material is a major scientific triumph for the mission. We are now optimistic that we will collect and return a sample with organic material – a central goal of the OSIRIS-REx mission,” said Dante Lauretta, OSIRIS-REx principal investigator at the University of Arizona in Tucson, in a statement.



Colder, Colder…
The process of sintering, or bonding the metals that make up the flexible circuits, usually happens at 572 degrees Fahrenheit.
“The skin surface cannot withstand such a high temperature, obviously,” Penn State engineer and lead author Hanyu “Larry” Cheng said in a press release. “To get around this limitation, we proposed a sintering aid layer — something that would not hurt the skin and could help the material sinter together at a lower temperature.”
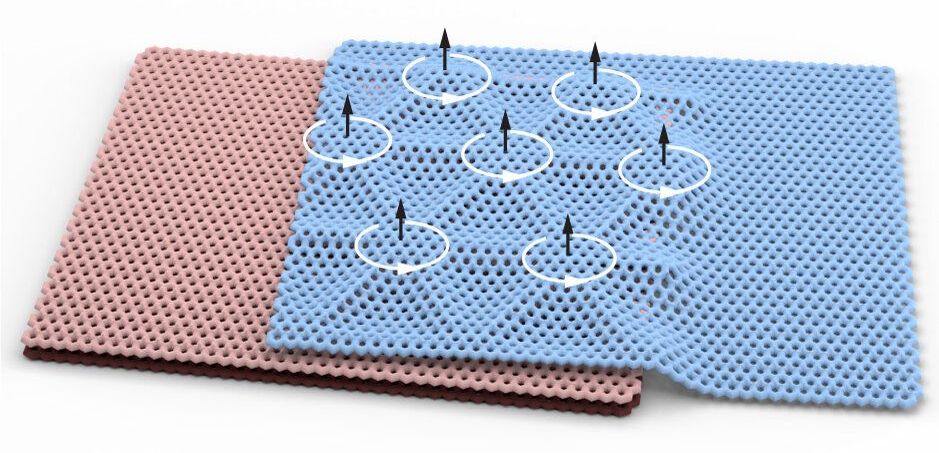
Since the discovery of graphene more than 15 years ago, researchers have been in a global race to unlock its unique properties. Not only is graphene—a one-atom-thick sheet of carbon arranged in a hexagonal lattice—the strongest, thinnest material known to man, it is also an excellent conductor of heat and electricity.
Now, a team of researchers at Columbia University and the University of Washington has discovered that a variety of exotic electronic states, including a rare form of magnetism, can arise in a three-layer graphene structure.
The findings appear in an article published Oct. 12 in Nature Physics.

NASA’s OSIRIS-REx Asteroid Sample Return Mission now knows much more about the material it will be collecting in just a few weeks.
Goddard’s Amy Simon found that carbon-bearing, organic material is widespread on the asteroid’s surface, including at the mission’s primary sample site, Nightingale, where OSIRIS-REx will make its first sample collection attempt on Oct.20.
These and other findings indicate that hydrated minerals and organic material will likely be present in the collected sample.
O,.o.
To solve a 100-year puzzle in metallurgy about why single crystals show staged hardening while others don’t, Lawrence Livermore National Laboratory (LLNL) scientists took it down to the atomistic level.
The research appears in the Oct. 5 edition of Nature Materials.
For millennia, humans have exploited the natural property of metals to become stronger or harden when mechanically deformed. Ultimately rooted in the motion of dislocations, mechanisms of metal hardening have remained in the crosshairs of physical metallurgists for more than a century.
Diamonds have a firm foothold in our lexicon. Their many properties often serve as superlatives for quality, clarity and hardiness. Aside from the popularity of this rare material in ornamental and decorative use, these precious stones are also highly valued in industry where they are used to cut and polish other hard materials and build radiation detectors.
More than a decade ago, a new property was uncovered in diamonds when high concentrations of boron are introduced to it: superconductivity. Superconductivity occurs when two electrons with opposite spin form a pair (called a Cooper pair), resulting in the electrical resistance of the material being zero. This means a large supercurrent can flow in the material, bringing with it the potential for advanced technological applications. Yet, little work has been done since to investigate and characterize the nature of a diamond’s superconductivity and therefore its potential applications.
New research led by Professor Somnath Bhattacharyya in the Nano-Scale Transport Physics Laboratory (NSTPL) in the School of Physics at the University of the Witwatersrand in Johannesburg, South Africa, details the phenomenon of what is called “triplet superconductivity” in diamond. Triplet superconductivity occurs when electrons move in a composite spin state rather than as a single pair. This is an extremely rare, yet efficient form of superconductivity that until now has only been known to occur in one or two other materials, and only theoretically in diamonds.