Molecular biology; Cell biology; Omics; Transcriptomics.


Human lifespan is shaped by both genetic and environmental exposures and their interaction. To enable precision health, it is essential to understand how genetic variants contribute to earlier death or prolonged survival. In this study, we tested the association of common genetic variants and the burden of rare non-synonymous variants in a survival analysis, using age-at-death (N = 35,551, median [min, max] = 72.4 [40.9, 85.2]), and last-known-age (N = 358,282, median [min, max] = 71.9 [52.6, 88.7]), in European ancestry participants of the UK Biobank. The associations we identified seemed predominantly driven by cancer, likely due to the age range of the cohort. Common variant analysis highlighted three longevity-associated loci: APOE, ZSCAN23, and MUC5B. We identified six genes whose burden of loss-of-function variants is significantly associated with reduced lifespan: TET2, ATM, BRCA2, CKMT1B, BRCA1 and ASXL1. Additionally, in eight genes, the burden of pathogenic missense variants was associated with reduced lifespan: DNMT3A, SF3B1, CHL1, TET2, PTEN, SOX21, TP53 and SRSF2. Most of these genes have previously been linked to oncogenic-related pathways and some are linked to and are known to harbor somatic variants that predispose to clonal hematopoiesis. A direction-agnostic (SKAT-O) approach additionally identified significant associations with C1orf52, TERT, IDH2, and RLIM, highlighting a link between telomerase function and longevity as well as identifying additional oncogenic genes.
Our results emphasize the importance of understanding genetic factors driving the most prevalent causes of mortality at a population level, highlighting the potential of early genetic testing to identify germline and somatic variants increasing one’s susceptibility to cancer and/or early death.
The authors have declared no competing interest.
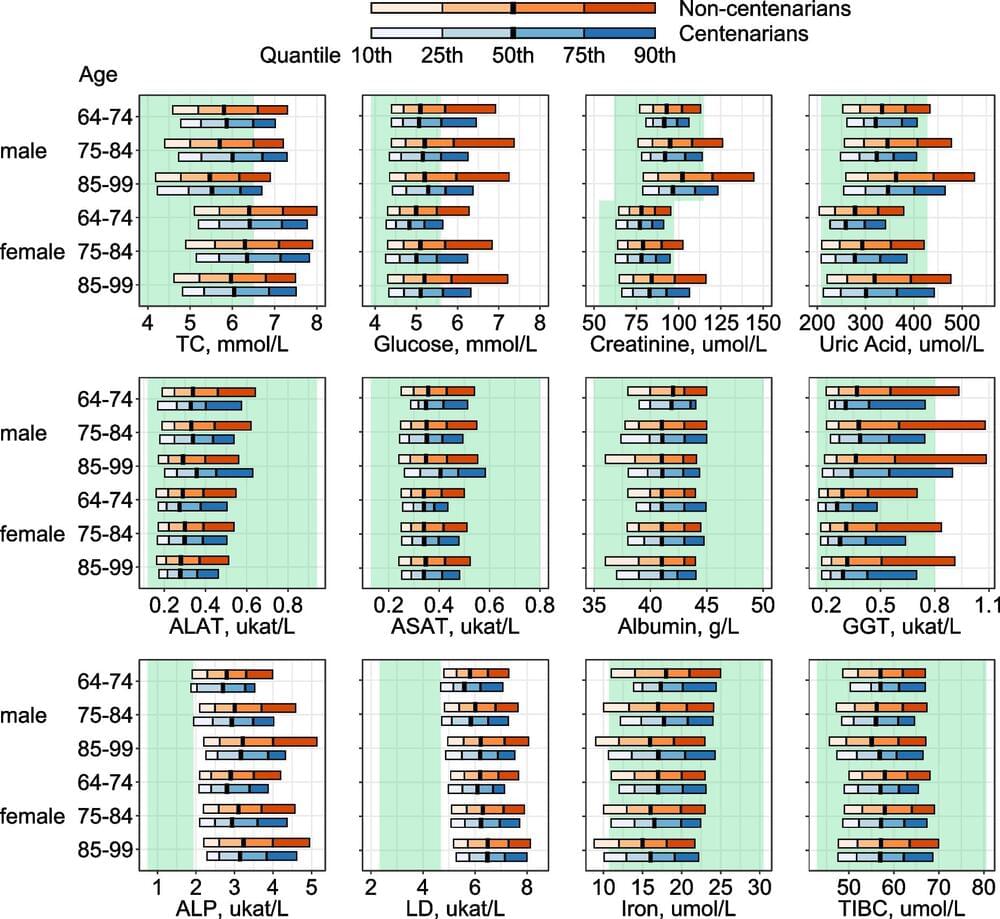
Knowledge of how centenarians’ biomarker profiles differ from those of non-centenarians at comparable ages already earlier in life is scarce. The lack of suitable, large prospective data with long follow-up is one likely reason for this. The Japanese cohort mentioned above included individuals aged 85+ only, and more than half of them were already centenarians at baseline enrollment. Since health selection likely starts even earlier than age 85, it is important to examine potential differences between long-lived individuals and those with average life spans already several years before—or during the process of—health deterioration.
Moreover, several studies have reported that centenarians are not such a homogeneous population as sometimes perceived. An Italian study based on 602 centenarians identified three subgroups with distinct health profiles [11]. It was found that 20% of the centenarians were in good health, 33% had intermediate health status, and 47% were in poor health. A Danish study also detected three distinct subgroups defined by health status: robust, intermediate, and frail centenarians [12]. About half of the Danish centenarians were in the “robust” group. A German study using health insurance data from 1,121 centenarians found four distinct comorbidity profiles, and only a small proportion of centenarians had a low morbidity burden [13]. These findings raise the question of whether such heterogeneity in centenarians’ health profiles is already visible earlier in life and, for example, reflected in their biomarker profiles. Uncovering potential heterogeneity in such profiles more than one decade ago may help us understand characteristics of health trajectories associated with exceptional longevity.
The AMORIS (Apolipoprotein MOrtality RISk) cohort offers a unique opportunity to compare biomarkers measured at similar ages but earlier in life between centenarians and their shorter-lived peers. The cohort contains a variety of biomarkers assessed approximately 30 years ago and was linked to several administrative health registers with data until 2020. Using these data, we aim to (i) describe biomarker profiles earlier in life among individuals eventually becoming centenarians and their shorter-lived peers, (ii) investigate the association between a set of biomarkers and the chance of reaching age 100 with up to 35 years of follow-up, and (iii) investigate differences in biomarker profiles within the centenarian population.




UC Merced researchers have found that the protein OTUD6 can alter protein production in cells, potentially affecting lifespan and cancer, with future research aimed at exploiting this for therapeutic benefits.
Researchers at UC Merced used fruit flies to uncover a cellular process shared by many organisms, which could significantly advance the understanding of cancer and aging.
Department of Molecular and Cell Biology Professor Fred Wolf, then-graduate student Sammy Villa, and Genentech Vice President and Senior Fellow in Physiological Chemistry and Research Biology Vishva Dixit, discovered a mechanism that cells use to tune how much protein they make through the process of translating RNA into protein.
Mind uploading and digital immortality explore the potential of AI technology to enable humans to live forever by transferring consciousness to machines. This concept raises profound questions about the future of humanity, identity, and ethics. Discover the groundbreaking possibilities and challenges of achieving eternal life through artificial intelligence and digital consciousness.
#ai #mindupload
