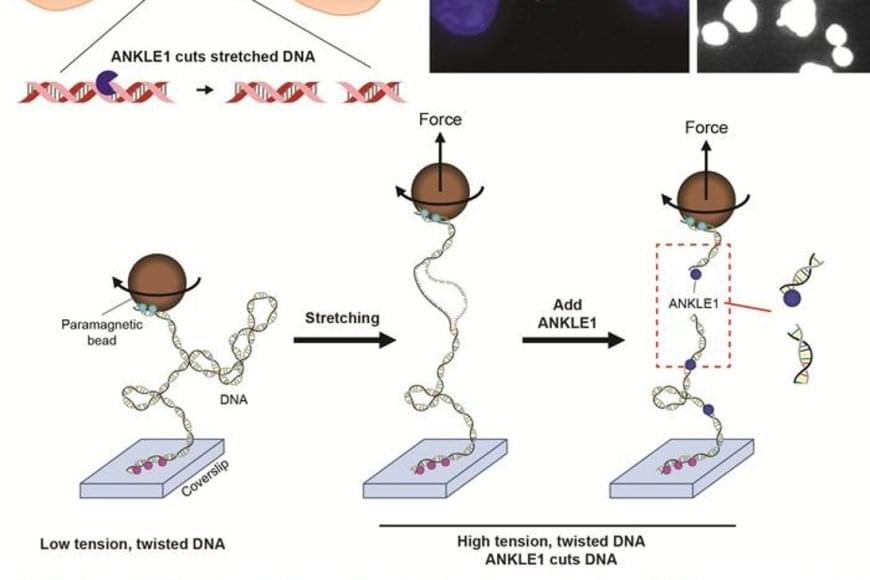
Scientists have long suspected that a small population of cells survives treatment and regenerates the tumor. These “cancer stem cells” are thought to resist conventional therapies, allowing the disease to return even after the visible tumor has been removed.
Previous attempts to identify gastric cancer stem cells using other protein markers, such as CD44 or CD133, yielded inconsistent results. These markers often appeared on healthy cells as well or did not fully account for tumor behaviour.
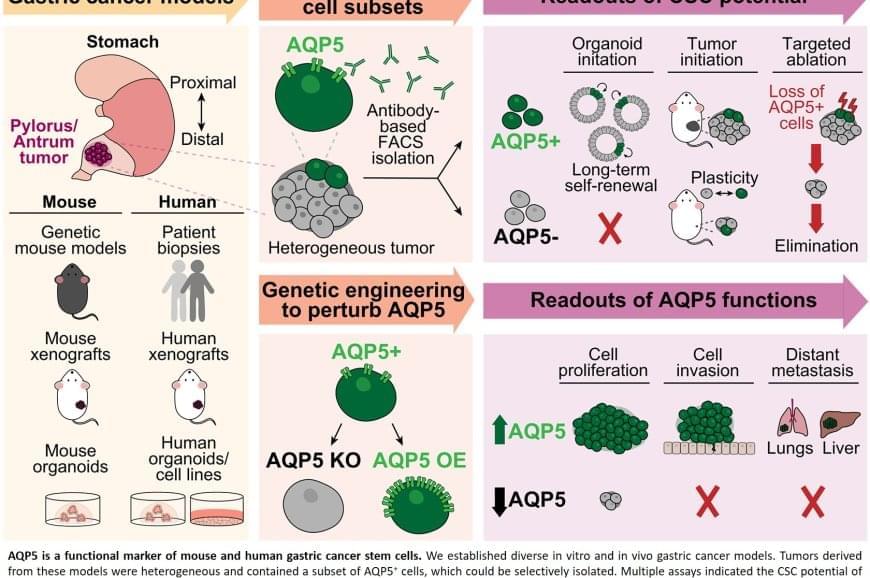
The team discovered that AQP5 reliably marks the cancer stem cells in gastric tumors. Aquaporins are proteins that form channels in cell membranes to control the movement of water into and out of cells. While AQP5 was previously known to mark stem cells in healthy gastric tissue, this study shows it also identifies the specific cells responsible for driving tumor growth, spread, and recurrence.
Importantly, AQP5 does more than simply mark these cells; it actively contributes to their aggressive behavior.
The researchers found that cells with AQP5 were capable of forming new tumors, while cells without AQP5 rarely did so. Most significantly, when they used a targeted method to eliminate only the AQP5-expressing cells, tumors stopped growing or shrank entirely and did not recur. This held true even for cancers that had spread to other organs.
Scientists have identified the specific cells responsible for gastric cancer’s tendency to return after treatment. The study also demonstrated that eliminating these cells stops tumors from growing, even in advanced disease that has spread to other organs.