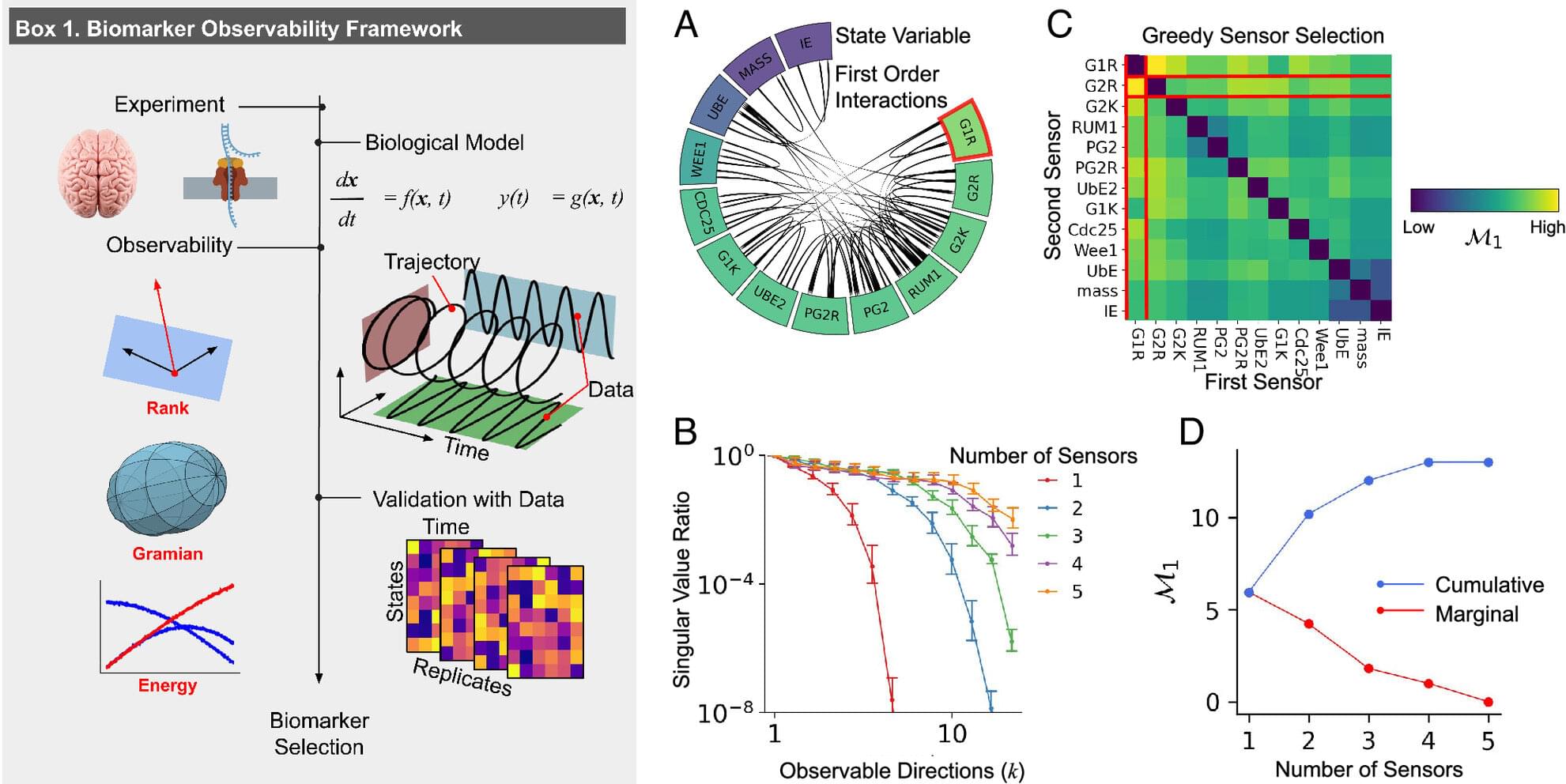
The cells in the human body are continuously challenged by a variety of genotoxic attacks. Erroneous repair of the DNA can lead to mutations and chromosomal aberrations that can alter the functions of tumor suppressor genes or oncogenes, thus causing cancer development. As a central tumor suppressor, p53 guards the genome by orchestrating a variety of DNA-damage-response (DDR) mechanisms. Already early in metazoan evolution, p53 started controlling the apoptotic demise of genomically compromised cells. p53 plays a prominent role as a facilitator of DNA repair by halting the cell cycle to allow time for the repair machineries to restore genome stability. In addition, p53 took on diverse roles to also directly impact the activity of various DNA-repair systems. It thus appears as if p53 is multitasking in providing protection from cancer development by maintaining genome stability.