Fortinet fixes critical FortiClientEMS SQL injection flaw (CVSS 9.1) enabling code execution; separate SSO bug actively exploited.



Efforts to unlock one of the mind’s greatest mysteries may get a boost from technology allowing us to capture dreams.
Welcome Friends to another Expanse video, this is an attempt to thread together everything we know about the Ring Builders history barring events in the last few books, this is speculative and based on my own thoughts and the opinions of others that make sense to me, just wanted to be up front about that.
You disagree with something, no bother let you voice be known in the comments.
Lets get straight into it.
In this episode of the Oncology Brothers podcast, we engaged in a thought-provoking discussion with Dr. Patrick Soon-Shiong, a pioneer in oncology and the innovator behind the approval of nab-paclitaxel (Abraxane). We delved into the exciting potential of Anktiva (N-803), an IL-15 receptor super agonist designed to expand and activate natural killer (NK) cells and CD8 T cells, with the hope of revolutionizing cancer treatment.
Dr. Soon-Shiong shared insights into the mechanism of action of Anktiva, its current approvals in non-muscle invasive bladder cancer, and extended approval in non-small cell lung cancer in Saudi Arabia, and the promising clinical trial data that suggests a significant increase in overall survival for patients. The conversation also touched on the importance of restoring lymphocyte counts and the implications for treating various tumor types.
Join us as we explore the future of immunotherapy, the challenges of regulatory approval, and the potential for Anktiva to change the landscape of cancer treatment.
Key Topics:
• Mechanism of action of Anktiva.
• Current approvals and clinical trial data.
• The role of lymphocyte counts in cancer treatment.
• Future directions for immunotherapy.
Follow us on social media:
• X/Twitter: / oncbrothers.
• Instagram: / oncbrothers.
• Website: https://oncbrothers.com/
Don’t forget to like, subscribe, and hit the notification bell for more discussions on the latest in oncology!
#Oncology #CancerTreatment #Immunotherapy #IL15Agonist #OncologyBrothers

Using ideas borrowed from topological photonics, researchers in Singapore, France and the US have designed a compact antenna capable of handling information-rich terahertz (THz) signals. Reporting their results in Nature Photonics, the team, led by Ranjan Singh at the University of Notre Dame, say that with further refinements, the design could help underpin future sixth-generation (6G) wireless networks, allowing data to be shared at unprecedented speeds.
In the not-too-distant future, 6G networks are expected to enable data rates of around one terabit per second—the same as transferring roughly half the storage of a mid-range smartphone in a single second. Achieving such speeds will require wireless systems to operate at terahertz frequencies, far higher than those used by today’s 5G networks.
However, before THz frequencies can be used reliably, major improvements are needed in the antennas that transmit and receive these signals.


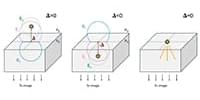
The University of Texas at Arlington researchers resolved a largely unnoticed modeling gap in how researchers interpret the behavior of single molecules at interfaces:
By treating emitters as finite-sized surface currents in contact with both media within finite-element simulations, this study provides the first physically self‑consistent framework for describing dipoles at arbitrary dielectric interfaces.
Spotlight by Matthew D. Lew.
A longstanding but largely unnoticed modeling gap has been skewing how researchers interpret the behavior of single molecules at interfaces—this work finally resolves it. Defocused fluorescence microscopy is widely used to infer molecular orientation, yet conventional models of dipole emission near refractive‑index boundaries diverge from one another depending on which side of the interface the molecule approaches. The result has been hidden, systematic biases in measured orientations. By treating emitters as finite-sized surface currents in contact with both media within finite-element simulations, this study provides the first physically self‑consistent framework for describing dipoles at arbitrary dielectric interfaces.