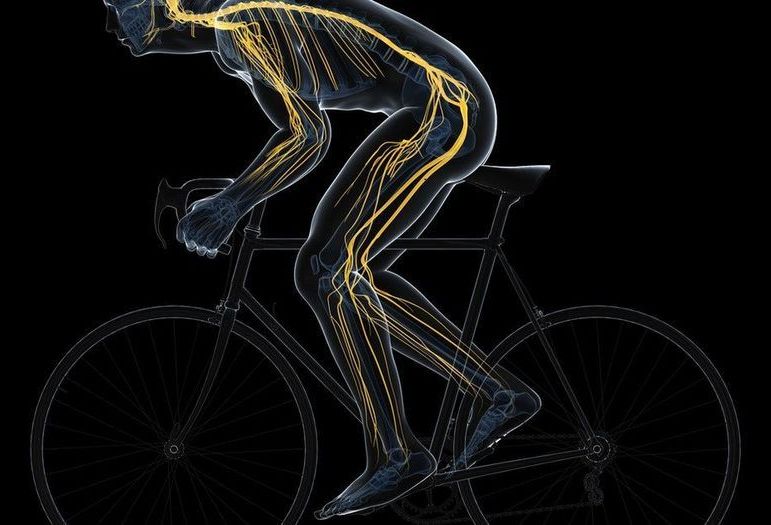
According to new research in mice, aerobic exercise may actually reverse aging’s toll on essential muscle stem cells.

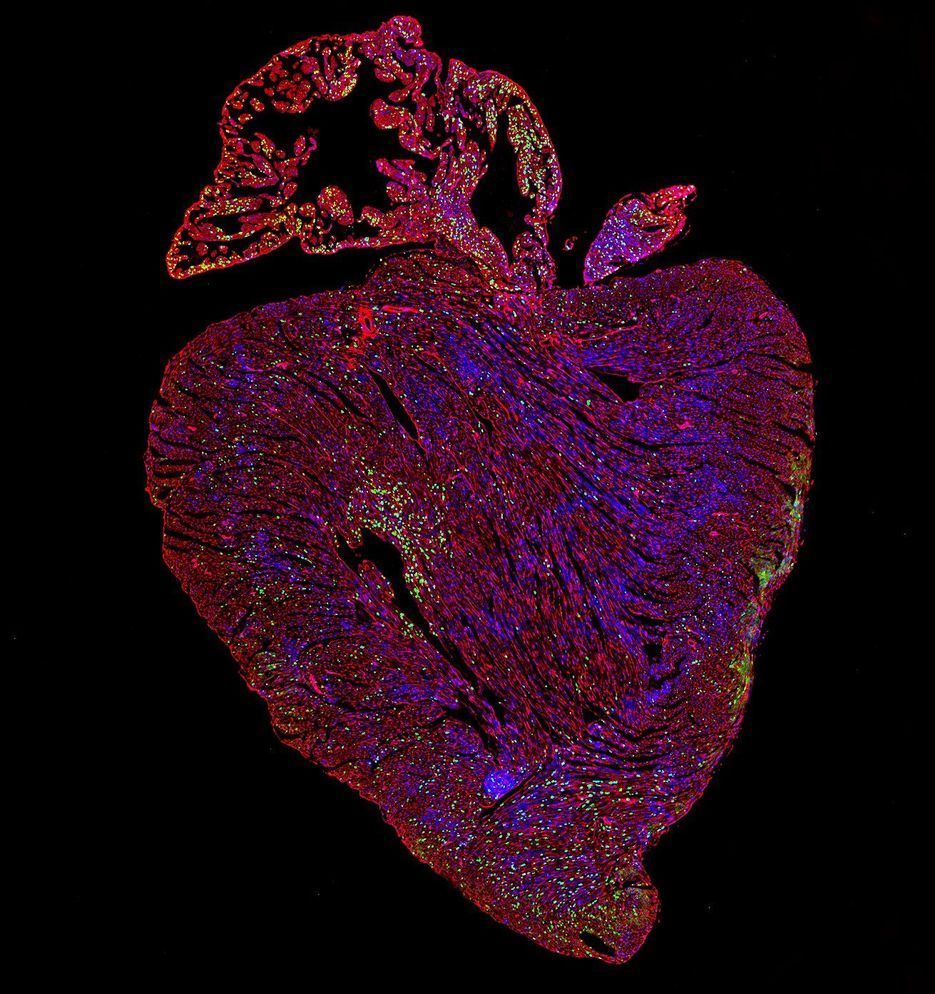
This is huge news… heart disease is the number one killer globally.
“They found that Myc-driven activity in heart muscle cells is critically dependent on the level of another protein called Cyclin T1, made by a gene called Ccnt1, within the cells. When the Ccnt1 and Myc genes are expressed together, the heart switches into a regenerative state and its cells start to replicate. The results are published today in the journal Nature Communications.”
Researchers trying to turn off a gene that allows cancers to spread have made a surprising U-turn. By making the gene overactive and functional in the hearts of mice, they have triggered heart cell regeneration. Since adult hearts cannot usually repair themselves once damaged, harnessing the power of this gene represents major progress towards the first curative treatment for heart disease.
“This is really exciting because scientists have been trying to make heart cells proliferate for a long time. None of the current heart disease treatments are able to reverse degeneration of the heart tissue—they only slow progression of the disease. Now we’ve found a way to do it in a mouse model,” said Dr. Catherine Wilson, a researcher in the University of Cambridge’s Department of Pharmacology, who led the study.
The cell cycle—through which cells make copies of themselves—is tightly controlled in mammalian cells. Cancer develops when cells start to replicate themselves uncontrollably, and the Myc gene plays a key role in the process. Myc is known to be overactive in the vast majority of cancers, so targeting this gene is one of the highest priorities in cancer research. Much recent research has focused on trying to take control of Myc as a means of cancer therapy.

In the midst of the coronavirus epidemic/pandemic it bears remembering the application of hyperbaric oxygen therapy to the last major pandemic that impacted the United States in 1918, the Spanish Flu Pandemic. Death was primarily by pulmonary infection and its attendant hypoxemia and respiratory failure. The first application of hyperbaric medicine to a Spanish Flu victim was likely also the first application to a human being in the United States. In 1918 Dr. Orval Cunningham of Kansas City was brought a dying friend of a fellow physician. The patient was moribund and blue. Before Cunningham could perform his planned animal experiments he was asked to treat this dying patient. With just a one-hour treatment with compressed air at 1.68 atmospheres absolute the patient experienced improvement. Combined with additional hyperbaric treatments over the next 3 days this patient’s life was saved. Others followed.
Today’s coronavirus’ mortality is due to pulmonary infection and respiratory failure. While there are differences between the Spanish Flu and coronavirus the primary pathology is in the lungs, the first organ of contact with hyperbaric therapy beyond the skin. The ability of hyperbaric oxygen to penetrate inflammatory pulmonary secretions allows adequate oxygen to reach the blood while inhibiting the inflammatory process. Applied correctly, hyperbaric therapy may have utility in coronavirus patients similar to its life-saving history with the Spanish Flu. Harch Hyperbarics Inc 504 309‑4948 www.hbot.com
Some doctors are questioning the way ventilators are being used for people with serious cases of COVID-19. Why? More data shows a high death rate for patients treated under current ventilator practices.
At the same time, these doctors are saying their patients behave more like they have high altitude sickness than a viral infection. They talk about two different types of COVID-19 patients with differing severe lung problems.
While some patients respond to treatment as expected, doctors also describe patients whose lungs seem relatively fine, but who still can’t get enough oxygen into their blood. These patients may make up the majority with severe infections.

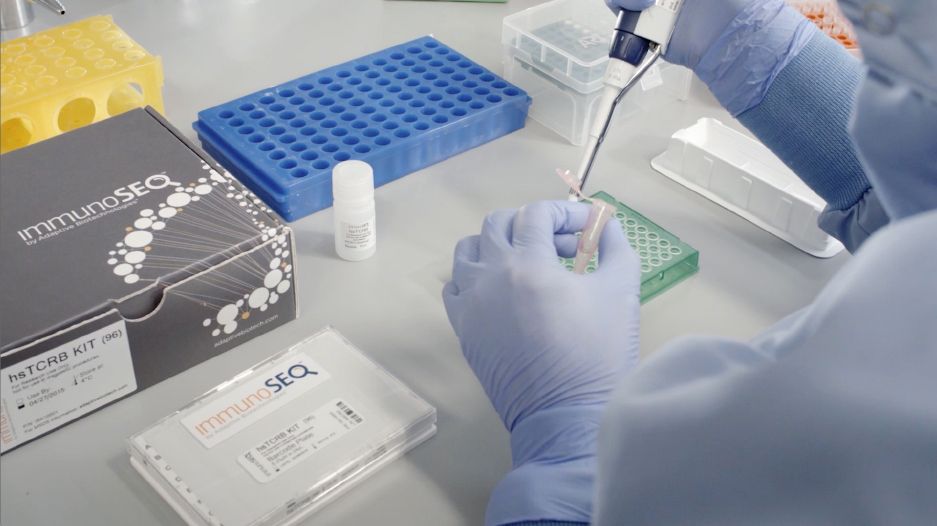
Despite a potential vaccine, the novel coronavirus could return on a seasonal basis, much like the flu, according to Seattle-based company Adaptive Biotechnologies.
That’s one of the reasons Adaptive is teaming up with pharmaceutical giant Amgen, which plans to use Adaptive’s proprietary technology platform to develop therapies to treat the virus.
Adaptive’s CEO Chad Robins says it’s the next big thing in the field of immune sequencing. They will screen blood samples of COVID-19 survivors, then identify which naturally occurring antibodies in the immune system can be used to neutralize the SARS-CoV-2 virus which causes the disease.

While Hokkaido was not covered in the state’s declaration of an emergency, the prefectural government and the municipal government of Sapporo, the prefectural capital, issued a joint emergency declaration following reports of double-digit increases in infections for the fifth straight day.
“We are facing a crisis of a second wave in the spread of (the coronavirus) infections,” Hokkaido Gov. Naomichi Suzuki told reporters, asking residents to refrain from making nonessential outings.
Hokkaido had declared its own state of emergency on Feb. 28 ahead of the government and lifted it on March 19, citing signs that the coronavirus spread was abating in the prefecture, a popular area for both Japanese and foreign tourists.

Talk of a universal basic income (UBI), or regular cash payments with minimal or no requirements for receiving the money, has been brought to the forefront as social distancing and economic concerns have put millions of people out of work.
Amid the COVID-19 pandemic Pope Francis says it might be time for some sort of universal basic income.
“This may be the time to consider a universal basic wage” to “acknowledge and dignify the noble, essential tasks” and to “achieve the ideal … of no worker without rights,” Pope Francis said in a letter to the World Meeting of Popular Movements, an organization representing global grassroots organizations, published on Sunday via the Vatican.
The Pope acknowledged that for many workers, the COVID-19 pandemic lockdowns are making it difficult, if not impossible, for people to earn money.

The coronavirus pandemic has resulted in the deaths of tens of thousands of people across the globe. It is also causing huge damage to the global economy. According to the predictions of the International Monetary Fund (IMF), 2020 could be the worst year since the Great Depression in the 1930s, with more than 170 countries likely to experience negative per capita income growth due to the pandemic. Countries are taking different measures to mitigate that economic impact, depending on the situations in their countries. However, the process of overcoming economic crisis is going to be extremely difficult. Few businesses would find it hard even to sustain and there is going to be a significant upsurge in unemployment rates.
Like much of the rest of the world, India is under lockdown. It is bit premature to predict the exact impact of coronavirus crisis on the Indian economy since the situation is still evolving. However, it is clear at this stage that the country will be facing major economic downturn and levels of unemployment will steeply rise. The government has already offered an economic package of 1.7 trillion rupees ($22.3 billion) in the last month for providing food security and money to the poor. It is expected that the government would shortly announce the next economic stimulus package.
All this would require the government of India to undertake a ruthless review of existing patterns of expenditure. The government budgeting caters to the requirements of various segments of the society, including agriculture, health, education, and railways. The budget has two other important areas of attention: defense and science & technology.

Scientists at the University of Alberta have shown that the drug remdesivir is highly effective in stopping the replication mechanism of the coronavirus that causes COVID-19, according to new research published today in the Journal of Biological Chemistry.
The paper follows closely on research published by the same lab in late February that demonstrated how the drug worked against the Middle East Respiratory Syndrome (MERS) virus, a related coronavirus.
“We were optimistic that we would see the same results against the SARS-CoV-2 virus,” said Matthias Götte, chair of medical microbiology and immunology at U of A.