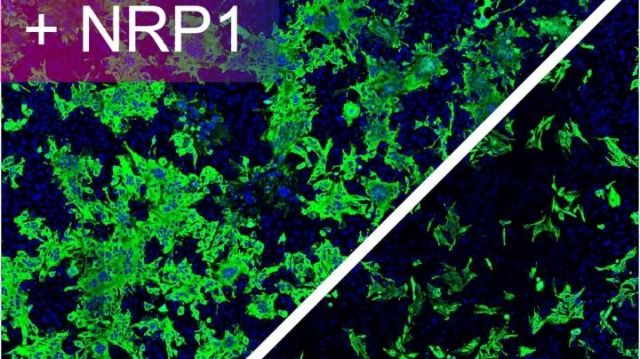
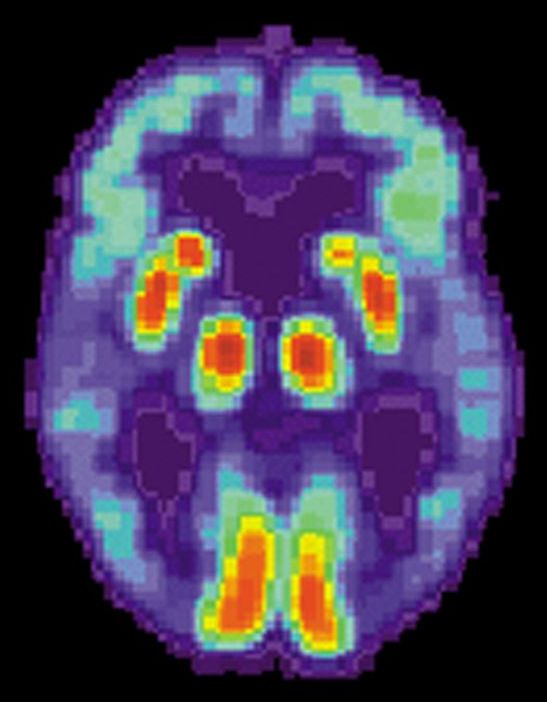
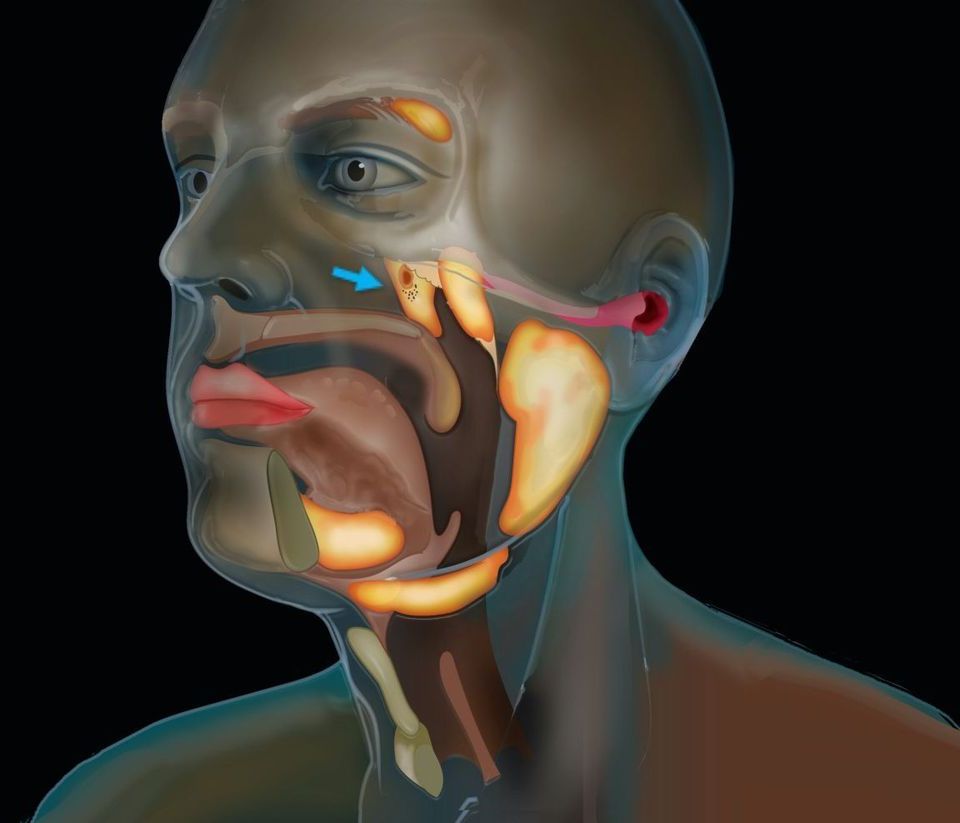
This includes volunteering to test the new vaccine being developed by the Israeli Institute for Biological Research in Ness Ziona. Meet some of the heroes who are doing their bit for humanity.
The research center said it will begin the clinical trials phase of its COVID-19 vaccine in November and about one hundred Israelis aged 18–55 are expected to participate by the time the trials are over. The first phase will include three brave volunteers and will take place in Sheba Medical Center in Tel HaShomer and Hadassah Medical Center in Jerusalem.
As with any other clinical trials, participants will be divided into two groups: those who will receive the real vaccine and those who will receive a fake vaccine, also known as placebo.