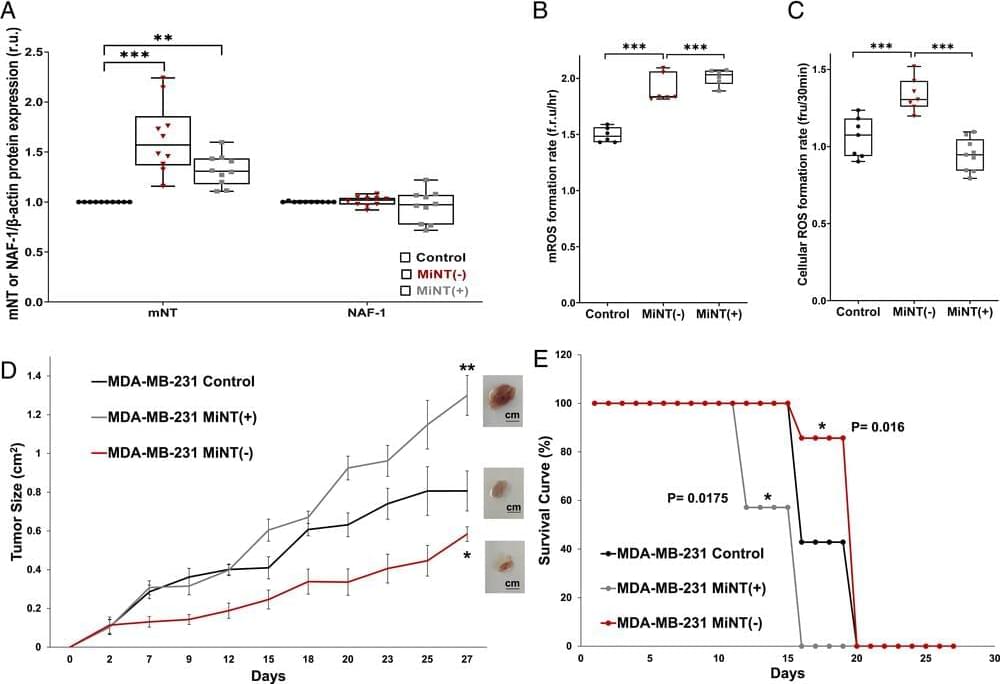
Here we address the important question of cross-talk between the mitochondria and cytosol. We show that the inner mitochondrial protein, MiNT, interacts with a protein on the outer mitochondrial membrane (mNT). This interaction occurs within the major outer membrane protein VDAC1. Inside the inner space of VDAC1, MiNT transfers its [2Fe-2S] clusters to mNT, which was shown to be a [2Fe-2S] cluster donor protein that donates its cluster(s) to apo-acceptor proteins residing in the cytosol. Hence, we suggest a pathway for transferring [2Fe-2S] clusters from inside the mitochondria to the cytosol.
Mitochondrial inner NEET (MiNT) and the outer mitochondrial membrane (OMM) mitoNEET (mNT) proteins belong to the NEET protein family. This family plays a key role in mitochondrial labile iron and reactive oxygen species (ROS) homeostasis. NEET proteins contain labile [2Fe-2S] clusters which can be transferred to apo-acceptor proteins. In eukaryotes, the biogenesis of [2Fe-2S] clusters occurs within the mitochondria by the iron–sulfur cluster (ISC) system; the clusters are then transferred to [2Fe-2S] proteins within the mitochondria or exported to cytosolic proteins and the cytosolic iron–sulfur cluster assembly (CIA) system. The last step of export of the [2Fe-2S] is not yet fully characterized. Here we show that MiNT interacts with voltage-dependent anion channel 1 (VDAC1), a major OMM protein that connects the intermembrane space with the cytosol and participates in regulating the levels of different ions including mitochondrial labile iron (mLI). We further show that VDAC1 is mediating the interaction between MiNT and mNT, in which MiNT transfers its [2Fe-2S] clusters from inside the mitochondria to mNT that is facing the cytosol. This MiNT–VDAC1–mNT interaction is shown both experimentally and by computational calculations. Additionally, we show that modifying MiNT expression in breast cancer cells affects the dynamics of mitochondrial structure and morphology, mitochondrial function, and breast cancer tumor growth. Our findings reveal a pathway for the transfer of [2Fe-2S] clusters, which are assembled inside the mitochondria, to the cytosol.