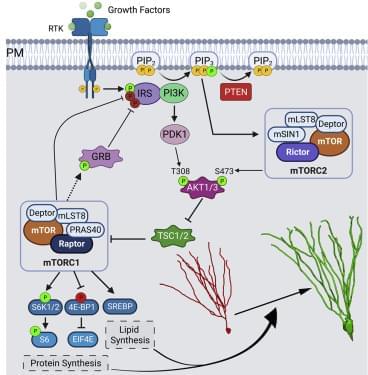
). At P7, Ptenflx/flx and Ptenflx/flxRaptorflx/flx animals were co-injected into the dentate gyrus with a retrovirus encoding a fluorophore (GFP) with a downstream Cre, and a control retrovirus with just a fluorophore (mCherry) and no Cre (Figure 3A). Here, the GFP-expressing newborn granule neurons are KOs for their respective flox genes, while mCherry-expressing neurons serve as their in-tissue WT controls. To investigate the role of mTORC1 in development of Pten KO-mediated somal hypertrophy, we quantified soma size of retrovirally infected immunolabeled granule neurons at P28. We observed that Pten KO neurons had significantly greater soma size when compared with their WT control. This increase in soma size was completely rescued in Pten and Raptor double knockout (DKO) neurons (Table S1A and Figures 3B and 3D). We further examined the role of mTORC1 in aberrant migration of Pten KO granule neurons. The Pten KO neurons migrate significantly farther from the hilus along the GCL, when compared with their WT control. This farther migration was completely rescued in Pten and Raptor DKO neurons (Table S1B and Figures 3B and 3E). The dendritic spine density was also found to be significantly increased in Pten KO neurons. This increase in number of spines in middle molecular layer was reduced to WT density in Pten and Raptor DKO neurons (Table S1C and Figures 3C and 3F). Additionally, the decrease in spine head diameter seen in Pten KO neurons was rescued in Pten and Raptor DKO neurons (Table S1D). However, the increased spine length of Pten KO neurons persisted in the Pten and Raptor DKO neurons (Table S1E). These data suggest that Pten loss-mediated neuronal hypertrophy can be rescued by targeting Raptor to disrupt mTORC1.
To examine the role of mTORC1 in the Pten loss-mediated dendritic overgrowth of granule neurons, we reconstructed and quantified retrovirally infected immunolabeled Pten KO granule neurons, as well as Pten and Raptor DKO granule neurons at P28 (Figures 4A and 4B). We observed that Pten KO granule neurons had more elaborate dendritic arbor. Sholl analysis revealed that Pten KO neurons had an increased number of intersections, when compared with WT control neurons. This increase was completely rescued in Pten and Raptor DKO neurons (Table S1F and Figure 4C). The total dendritic length was also increased in Pten KO neurons, which was rescued in Pten and Raptor DKO neurons (Table S1G and Figure 4D). Further analysis revealed that Pten KO neurons have more primary dendrites protruding directly out of the soma, when compared with their WT control. This increase in number of primary dendrites was completely rescued in Pten and Raptor DKO neurons (Table S4A and Figure S2A).