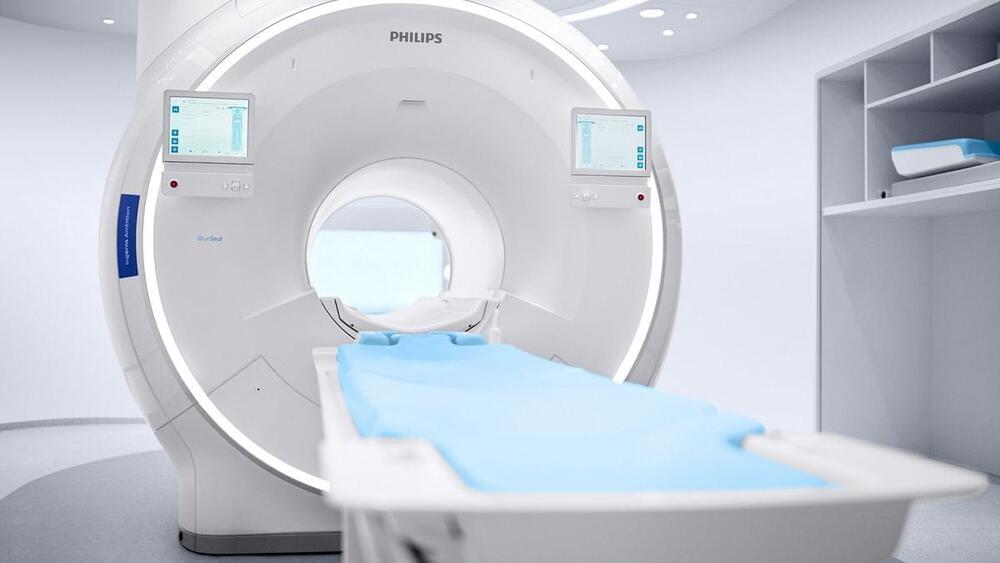
The artificial intelligence software speeds up the process of taking scans.
Philips received clearance from the FDA for its artificial intelligence MR platform that is used to detect cancerous tumors in the head and neck.
FDA clearance for AI technology
The company announced that the FDA gave clearance for Philip’s AI-enabled MRCAT radiotherapy. The clearance, also known as the 510(k) clearance, requires device manufacturers to register, and notify FDA of their intent to market a medical device at least 90 days in advance.