The main structure shaping the mouse digit tip in size and form is the terminal phalangeal bone (Fig. Supl. 1). As all long bones, the terminal phalanx is formed during embryonic development through endochondral ossification, resulting in longitudinal growth, and through the process of appositional ossification, resulting in peripheral growth13. Differently from all long bones, the length of the distal phalanx is further increased by an additional ossification center located at the distal tip of the bone, through intramembranous ossification13,14. Estimations point that 55% of the postnatal elongation of the distal phalanx of mice is a consequence of this distal process13. One study showed that although distal amputation eliminates part of the terminal phalanx formed by endochondral ossification, bone regrowth after amputation is exclusively due to distal intramembranous ossification13. Similar to bone formation by intramembranous ossification during development or after injury16, bone regrowth after distal amputation of the mouse digit tip depends on Wnt signaling17.
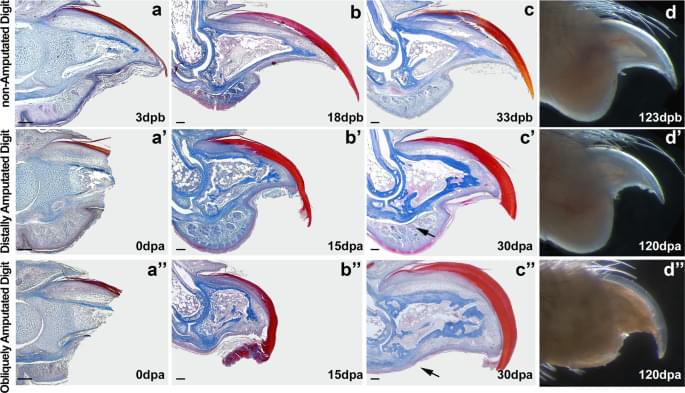
Re-establishment of homeostasis and nail and bone regrowth are expected to occur naturally after digit tip lesions. However, the formation of a blastema, which is a hallmark of epimorphic regeneration in salamanders, has been largely discussed in mammals7,9,18,19,20,21,22. The search for a mammalian blastema is sustained by the idea that, as in salamanders, tissue-specific cells, or stem/progenitor cells, would respond to the distal amputation lesion, migrating to a central distal-most region of the digit, creating the multi-tissue structure comprising the regenerating digit. A study in mice17 shows that as in salamanders, each tissue comprising the regenerated digit is formed by tissue-specific stem cells residing in the tissues preserved after amputation, suggesting that trans-differentiation does not occur in amputated mouse digit tip. However, it is not known whether these stem cells are integrating a regenerative blastema induced by the distal amputation or simply generating more tissue through endogenous tissue repair responses.
In this study, we compared digit regenerative capacity after distal and proximal amputation and propose a hypothetical mechanism by which digits are able to regain a morphology that is close to normal after distal amputations but fails when amputations are performed proximally. While most tissues re-establish homeostasis in very similar ways in distal and proximal amputated digits, bone growth is only observed after distal amputations. Observing the regions affected by each amputation plan, we propose that the main difference between these two amputation plans is the elimination of osteogenic signals and precursor cells in proximally amputated digits. In distally amputated digits, the source of osteogenic signal emanating from the nail17 and the presence of osteoprogenitor cells in the periosteum23 could be sufficient to promote bone growth and give the digit a new tip.