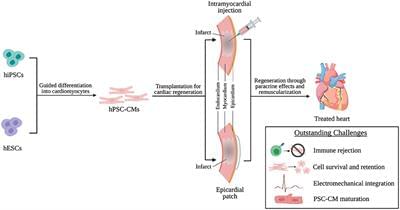
Cardiovascular disease is the leading cause of death worldwide and bears an immense economic burden. Late-stage heart failure often requires total heart transplantation; however, due to donor shortages and lifelong immunosuppression, alternative cardiac regenerative therapies are in high demand. Human pluripotent stem cells (hPSCs), including human embryonic and induced pluripotent stem cells, have emerged as a viable source of human cardiomyocytes for transplantation. Recent developments in several mammalian models of cardiac injury have provided strong evidence of the therapeutic potential of hPSC-derived cardiomyocytes (hPSC-CM), showing their ability to electromechanically integrate with host cardiac tissue and promote functional recovery. In this review, we will discuss recent developments in hPSC-CM differentiation and transplantation strategies for delivery to the heart. We will highlight the mechanisms through which hPSC-CMs contribute to heart repair, review major challenges in successful transplantation of hPSC-CMs, and present solutions that are being explored to address these limitations. We end with a discussion of the clinical use of hPSC-CMs, including hurdles to clinical translation, current clinical trials, and future perspectives on hPSC-CM transplantation.
Cardiovascular disease (CVD) is the leading cause of death worldwide. In the United States alone, CVD is responsible for ~655,000 deaths and contributes to $200 billion in spending each year. CVD can lead to myocardial infarction (MI), also known as a “heart attack,” which results in restricted blood flow and extensive cell death within the infarct zone. Due to the limited regenerative capacity of the human heart, infarcted myocardium is replaced by fibrotic scar tissue with inferior contractile performance. Over time, pathological remodeling leads to ventricular wall thinning, which can progress to heart failure. There is currently no treatment available that can restore lost cardiomyocytes after MI, and conventional therapies typically only manage the symptoms (3, 4).