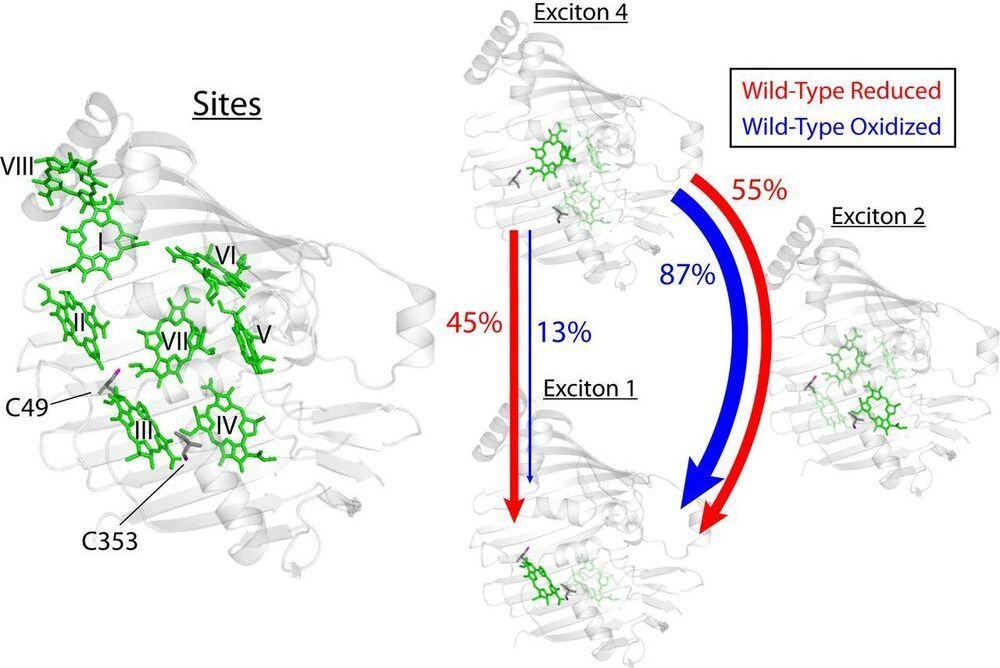
Photosynthetic light-harvesting antennae transfer energy toward reaction centers with high efficiency, but in high light or oxidative environments, the antennae divert energy to protect the photosynthetic apparatus. For a decade, quantum effects driven by vibronic coupling, where electronic and vibrational states couple, have been suggested to explain the energy transfer efficiency, but questions remain whether quantum effects are merely consequences of molecular systems. Here, we show evidence that biology tunes interpigment vibronic coupling, indicating that the quantum mechanism is operative in the efficient transfer regime and exploited by evolution for photoprotection. Specifically, the Fenna–Matthews–Olson complex uses redox-active cysteine residues to tune the resonance between its excitons and a pigment vibration to steer excess excitation toward a quenching site.
Photosynthetic species evolved to protect their light-harvesting apparatus from photoxidative damage driven by intracellular redox conditions or environmental conditions. The Fenna–Matthews–Olson (FMO) pigment–protein complex from green sulfur bacteria exhibits redox-dependent quenching behavior partially due to two internal cysteine residues. Here, we show evidence that a photosynthetic complex exploits the quantum mechanics of vibronic mixing to activate an oxidative photoprotective mechanism. We use two-dimensional electronic spectroscopy (2DES) to capture energy transfer dynamics in wild-type and cysteine-deficient FMO mutant proteins under both reducing and oxidizing conditions. Under reducing conditions, we find equal energy transfer through the exciton 4–1 and 4–2–1 pathways because the exciton 4–1 energy gap is vibronically coupled with a bacteriochlorophyll-a vibrational mode.