One step closer to a light into matter molecular synthesizer: 3.
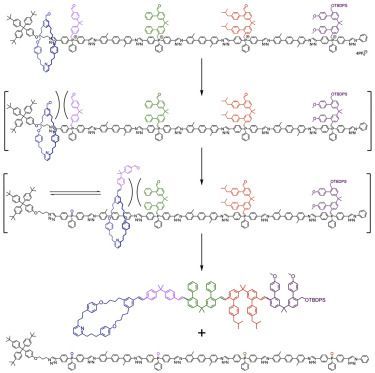
Molecular machine-track conjugate 1 (Figure 1) was designed to use iterative Wittig reactions to form carbon-carbon double bonds between a macrocycle and building blocks abstracted one at a time and in sequence from a track. The Wittig reaction 24, 25, 26 was chosen as it is robust and structurally tolerant, lending itself to exploitation in a range of contexts, including dynamic DNA-template synthesis.9 Our machine is based on a rotaxane architecture, in which the macrocycle has a reactive aldehyde attachment and the axle has the building-block sequence encoded as phosphonium salts during its synthesis. The 2, 2-diphenylpropane phosphonium units act both to restrict the position of the ring on the track and, upon deprotonation, as reactive ylide functionalities. Each ylide is large enough to block the passage of the macrocycle, trapping the ring within a compartment defined by the bulky stopper at the terminus of original threading and the next ylide along the track. Once a reactive building block can be reached by the macrocycle-appended aldehyde, it can be removed from the track through a Wittig reaction that adds it to the terminus of the growing chain. Each barrier also contains an aldehyde unit, so that once the building block is added to the end of the chain, it is able to react with the next barrier on the track that the macrocycle can access, enabling the alkene-connected oligomer to grow through successive Wittig reactions.
The specific size and constitution of the 2, 2-diphenylpropane motif of the building blocks proved important for successful machine operation. Early track designs in which the ylide and aldehyde were attached to the same aromatic ring or extended conjugated system proved insufficiently reactive (see Section S7 for a brief discussion of initial designs). Embedding the phosphorus atoms within the vector of the track allowed synthetically accessible triaryl phosphines to be the basis of the track design, expediting the synthesis (see Sections S2 and S3). The phenyl substituent at each phosphorus center (e.g., 4a–4D) also proved important: when a tolyl (4-methylphenyl) linking group was investigated, it proved difficult to develop macrocycles that could both thread during the rotaxane-forming reaction and, subsequently, pass over the phosphine oxide in the track formed from the Wittig reaction.
Each phosphorus center is attached to a methylene group bearing a diarylpropane building block derivatized with a different pair of substituents (H, Ph, C6H4CH2CHMe2, or C6H4OMe). These provide different sidechains in the machine product, the same role that different amino acids play in proteins. However, two (identical) sidechains are present per monomer using this artificial molecular machine design compared with one sidechain per amino acid in proteins. This was chosen partly to illustrate how artificial machines and their products are not subject to the same constraints as biomolecular synthesizers but, conveniently, the symmetry of the building blocks also makes their synthesis more straightforward. Each phosphonium moiety is separated from the next by rigid spacers that prevent folding of the track and so ensure that the phosphonium salts can only react with the aldehyde group at the end of the chain attached to the macrocycle rather than others on the track.